4.2 New Product Development
One area where the success of R&D was most evident was the impact of ‘new’ products on SOLA’ sales line.
| Click on images to enlarge: | ||||
|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
SOLA was successful at utilising the different skills and cultures of the personnel in the SOLA world, e.g. successful group technical conferences were held in Lonsdale during the 1980s that brought together representatives from all over the SOLA world. The conferences were used to disseminate new product and process information and to coordinate technical effort carried out in the SOLA technical centres (Europe, USA and Australia) and at the casting sites.
Some examples of the changing R&D priorities from 1989 to 2005 follow:

In 1989/0, with Peter Coldrey as director, ~50% of the R&D budget was devoted to new products. (From ~1985 to 1995, SOLA’s total expenditure on R&D was ~3.5% of sales.) The remaining activities were new processes, product development, direct support, process development and strategic QC. The main projects were:
- Spectrum
- Desensitize casting process
- RENI (manufacturing cost reduction - precursor to Rooster)
- Strategic quality analysis
- Instrument manufacture
- Coating development
- Direct support
- High refractive index polymers
- New ophthalmic monomers and polymers
- Tinting and colour chemistry
- Glass progressives
- Resin lens design
- CAD of lenses
- Alternate mold material and manufacture
- Stock lens power
In October '92, Colin Perrott introduced the A-teams concept. The thought was that instead of just talking about products SOLA should talk about lens design, materials and coatings, etc. A-teams brought together the best people in the different facets of the company from marketing, R&D and Production to make it happen. Peter Coldrey in the USA and Matthew Cuthbertson in Australia then developed a number of techniques, particularly project briefs, to allow the development of, or the writing down of, the concepts which were behind the programs. Nothing got done unless there was a project brief. So SOLA went from “you have to have a customer” to “you have to have a customer plus a clear definition of what it is you want to do”, even if that's a strategic type development.
In reality, the A-teams only worked where there already was a strong vested interest in the activities that the teams were trying to pursue. It reality, it meant that when it got down to the wire, for any program to be successful there has to be an absolute champion for the program who would just push it through. Typically that champion needed to be someone commercial; not just someone in the technical organization.
In 1992/3, with Matthew Cuthbertson as director, Australian R&D was working on the following projects:-
New Products
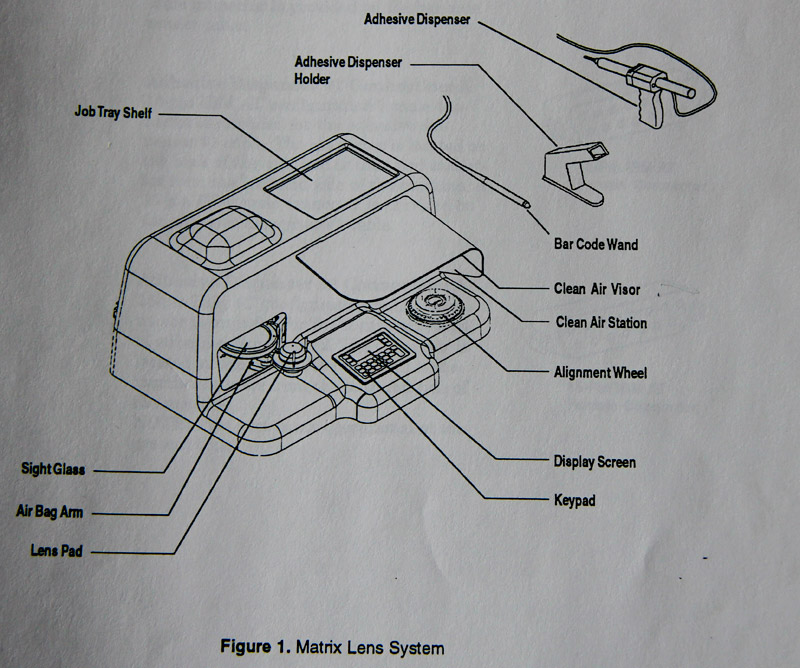
- Matrix lens system
- New Spectralite products
- High Index XL progressive
- New product feasibility/support
Process Technology
- Spectralite cost reduction
- Project Q (a mixture of process and quality improvements)
- Operation Copycat (precursor to Rooster)
- Major CR-39 process improvement
- Machining aspheric surfaces
Marketing and Operations Support
- Technical marketing support
- Powermetres
- Strategic Quality and National Standards
- Direct support
New Opportunities
- Vision research/lens design
- Speciality planos
- Plastic photochromics
- New 1.6 high index lens
- AR coating by magnetron sputtering
- Exploratory research
Mathew brought a very disciplined and structured approach to R&D. In developing R&D Australia’s strategic plan, he generally followed Ted Gioia’s standard format, namely:-
- Mission statement
- Summary of recent changes
- Analysis of Strengths/Weaknesses/Opportunities/Threats
- Current goals and priorities
- 3 year milestones
Another critical point was teamwork and excellent cooperation and working relationship at all levels with SOLA USA R&D, then under the leadership of Peter Coldrey.
In 1995, SOLA USA’s stated objective was “within 5 years, about half of our sales will come from products that don’t exist today”. However it is even more instructive to examine the sustained impact of products released since the Graduate/VIP. Over 70% of 1995 sales dollars were derived from products released during that period (1984 – 1995); and that trend was seen across all regions and key business sectors.
The Corporate plan in 1996 stated “SOLA will be R&D orientated in its overall strategy. Its marketing success and profitability will rely heavily on product innovation, efficient manufacturing methods and high quality.”
By 2000, the emphasis had shifted to the following projects:-
TOP 10 PROJECTS
- Goldfish
- Poly Matrix
- Global Specifications
- In-Mould Coating
- FIST
- SOLA MAX in Spectralite
- Progressive Lens Development
- Project Ibis/Spectralite Transitions IV
- AO Compact in Polycarbonate
- Antistatic UTMC
- Mini Rooster
OTHER PROJECT – AUSTRALIA
- Retail Support
- Graphical Concepts and Techniques
- Finished MC Progressive
- Lens Design Software Development
- Vision Research
- WideEyes
- Spazio/ASL CR-39 for Sun Rx
- R&D Intranet
- Instrument Measurement Support
- Technical Marketing Support
OTHER – USA
- Progressive Design for MODC
- PPG 1080 Development
- Technical Marketing Information Library
- Technical Marketing - Individual Testing of Competitive Product Entries Versus
- Comparable SOLA Products
- Strategic Evaluations – Benchmarking
- DSI Process Improvement Project
- AXXICON/Finished Poly Moulding
- Back Surface Hardcoat
PROJECTS – AO
- AO Compact
- AO Fashion-wear Options
- AO Next Generation Progressive
- OEM Project
During this period (2000), due to the need for substantial operating expense reductions, the R&D headcount was reduced by a total of 14 people out of a total of approximately 90.
By FY2001, value added products equalled 68% of revenues but only 21% of production volume. The make-up of net sales:-
- 41% progressives
- 27% single vision value added (lens coatings, photochromics, thinner and lighter, Rx lab services)
- 27% single vision, bifocals (standard plastic and glass commodity)
- 5% other (plano lenses and sunlens)
... with global diversification of
- North America 44%,
- Europe 33% and
- ROW 23%
The New Products report of September 2002 stated:
INTRODUCTION
New Products have been key drivers of SOLA and AO’s growth over the last 15 years. To ensure this is sustained, SOLA International continues to invest in new products and technologies, with a focus on those that meet consumer vision and lifestyle needs.
The thrust of our product and marketing effort is to have innovative and differentiated offerings with easy to understand positioning and communications for the retailer and the consumer.
This product strategy focus has encompassed both the SOLA and AO brands.
TODAY
A combination of challenges in the market required us to restructure the way SOLA and AO did business. As part of the restructuring, the New Product program was overhauled to provide more balance in the management of the product portfolio. This ensured projects were balanced across strategic initiatives, some new “conventional” products such as new general purpose progressives, as well as product/sku rationalization of older, less viable products. This increased focus on a handful of key strategic and tactical initiatives also improved the alignment to the business to ensure stronger commercial performance in the future.
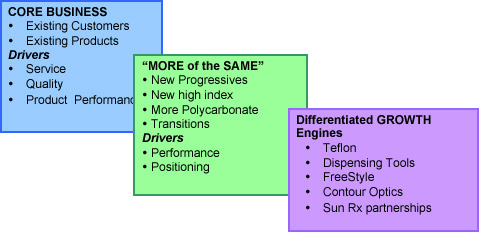
KEY ELEMENTS OF PRODUCT STRATEGY

BASIC PREMISES UNDERPINNING PRODUCT PROGRAM
It is essential that SOLA and AO maintain core business and leverage every opportunity for organic growth from our existing customer base using existing products and distribution capability.
The introduction of “conventional” new products such as a new progressive or another high index product can be easily accommodated using existing infrastructure and capability without distracting from the core business. These are essential to freshen our offering to minimally maintain our core business but potentially to grow share and increase margins.
The introduction of new innovative products or delivery vehicles such as Contour Optics and FreeStyle change the paradigm and require a level of sophistication overlaying the existing organization and infrastructure to ensure we maintain the output of the core business and use the new initiatives as growth engines to achieve incremental business.
LAUNCH PRIORITIES
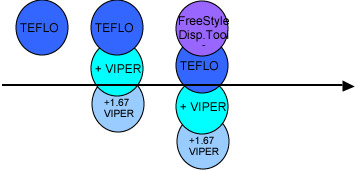
Teflon will be launched in all markets by the end of FY03. This will continue to be the main marketing focus for all regions for at least the next year.
This has been coupled with regional marketing and promotional effort to boost the sales of SOLAMAX and AO Compact. This has been successful and the intent is to maintain the momentum and not dilute it with a VIPer progressive launch in the main market.
VIPer will be launched as an Rx product only in the major markets. The product will be configured as the best new general purpose progressive available with the best treatment on the market, “Teflon”. This will allow us to keep Teflon in the limelight. It also provides an opportunity to introduce our first freeform offering with the 1.67 VIPer.
FreeStyle as a new business comes into its own with the role out of dispensing systems and the ability to create the marketing pull-through for customized products. This will be developed and marketed over time.
KEY NEW PRODUCT INITIATIVES
| CATEGORY | STATUS |
|---|---|
| New Progressives |
VIPER New general utility progressive lens design for implementation under the SOLA brand. The product will principally be sold via our own Rx labs except in South America, and select parts of Asia and Australia. AO ProEasy New general utility progressive lens design for implementation as a mass market product under the AO brand. Replacement for AO Pro but has potential to shore up VIP/XL business in the North American market. |
| Expanded 1.67 offering | The market is increasingly moving to higher and higher index product usage. Establishing an in-house capability to produce 1.67 “hockey pucks” for FreeStyle processing and implementation expands our opportunity to participate in this segment via directly generated progressives, atorics, aspherics, etc. |
| Teflon | Teflon will be launched in all markets by the end of FY03. The initial indicators are very exciting and have led to a rapid capacity expansion. This offers the potential to grow not only SOLA and AO’s share of an existing category but also grow the overall category substantially. Select chain retailers in each geographic location will be able to utilize the Teflon technology in partnership with SOLA. |
| Dispensing Tools |
Dispensing Systems such as the PPS head-tracking system used by Grand Optical can facilitate significant growth in the sale of premium products creating a corresponding demand from SOLA and AO. A number of hardware and software components are being developed to create a “kit bag” of dispensing tools to mix and match for different customers and distribution channels. These “dispensing systems” will be tailored and implemented by customer within some agreed commercial framework |
| FreeStyle |
FreeStyle embraces the manufacture and delivery of optimized and/or customized products. What differentiates this from other freeform technology platforms and products is the effort on the front end dispensing process with both the customer and the retailer in mind. This concept also relies on some changes in Rx competency and behaviour that are currently being implemented for the branded Sun Rx programs to present virtual shop fronts for a range of customers. A number of freeform generators and polishing systems are being purchased and delivered over the next few months and this will establish a base capability in a number of our labs to deliver optimized/customized products. |
| Contour Optics |
Discussions with a number of potential frame and brand partners are progressing well with the potential for some agreements to be in place by November. A test market with a new partner is planned for June’03. |
| Sun Optics | A platform of new products along with an increasingly global capability to process ophthalmic product with various sunlens treatments is forming the foundation of a new strategic initiative to develop Sun Optics sales in the Direct to Retail business. Businesses such as SOI and AOF already have strong Sun Rx programs but this expands that capability to provide virtual shop fronts for key partners such as Nike, Bolle, etc. |
In 2003, with Simon Edwards as director, the primary R&D projects for SOLA and AO were:-
- VIPer (SOLAOne)
- Teflon
- Freeform Technology Implementation
- Sun Rx Implementation
- AO Compact Poly Transitions
- AO ProEasy/PEZ
- Face Form Lens
- SOLAMax
- Polycarbonate Flat Top 28 Lens Product Development
- OSM Poly S/F Process Windows
- Thermoplastics Processing Development
- S/F Poly Spazio
- Polycarbonate UV Coatings (Acrypol)
- Rx Coatings
- Product Evaluations
- Instrument and Measurement
- Specifications and Standards
- SOLAnet Development
- Autoplotter Replacement
- Teflon Cost Down
- Vision Research
- PAL Technologies
- INSIGHT Development
- Next Generation Contour and Wrap Optics
- CRC for Polymers
- Next Generation Photochromics
- Coatings Technology Development
- Business Opportunities and IP Strategies
- Strategic Materials
- Thermoplastic Materials
- Ink Technology Development
- Super ISR
| R&D Employees | ||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
After 2005, R&D control shifted to Germany.
SOLA - A MARKET LEADER? Karen Roberts
In many ways, SOLA was a company ahead of its time. The company was capable of quickly developing and launching new products, and in many cases was the first to invent, ultimately creating many sub categories that are now a mainstay of the broader market place.
I started my career at SOLA as it was launching the VIP/Graduate progressive into an emerging progressive lens market. SOLA and its subsequent subsidiary American Optical (AO) went on to offer some of the leading progressives in the market with the first ‘Design by Rx’ lens, the first ‘Soft progressive lens’, the first ‘Short corridor’ design for small frames, the first ‘Active’ lens designed for good peripheral performance, the first ‘Lifestyle’ customised progressive, to name just a few.
In addition to the lens design prowess of SOLA and AO, SOLA was also a market leader for many years in developing and implementing new innovative lens materials and coatings:
- SOLA launched the first high index, high ABBE material on the market; Spectralite.
- focus on ABBE created a new awareness in the market and all subsequent material launches included some reference to ABBE in recognition that this was an important criterion in the performance of all new material.
- Spectralite (or the Spectrum technology foundation) allowed SOLA to go on and offer a number of highly flexible material configurations and products.
- One of these was the development of “Sensilite”, a high performance photochromic technology that was used in subsequent generations of photochromic product offerings.
- Spectralite was the host for the fastest photochromic in the market, Spectralite Transitions III, and its successor Spectralite Velocity.
- These outperformed their counterparts in standard Transitions portfolio and were valuable contributors for both Transitions and SOLA for many years. The last generation of Transitions product, Transition VI, has finally caught up to Velocity in activation and fade-back performance, a mere five years after it was first launched.
- SOLA also developed and launched the first true 1.60 index material with a high ABBE performance, continuing to build on the foundation of Spectralite’s good optical characteristics.
- SOLA developed and offered the first anti-static Anti-reflective coating in the market, eventually marketing this product under the licensed brand name, Teflon.
- Teflon was revolutionary in that it was a paradigm changing Anti-reflective coating with a superior transmission performance, superior cleaning attributes and tough anti-scratch performance. These CLEAR, CLEAN, TOUGH attributes combined with a blue reflex hue soon became the benchmark in the industry with all major competitors offering a like product.
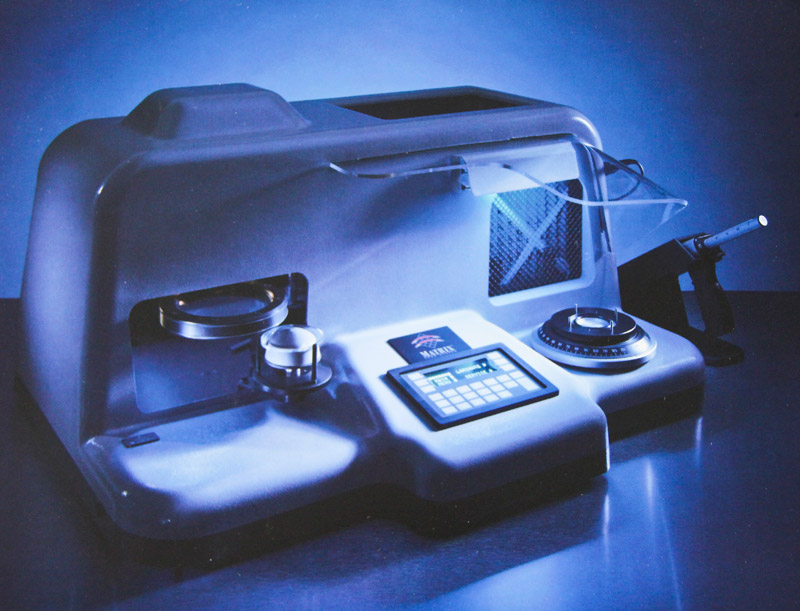
SOLA was a pioneer in in-office manufacturing systems for 1 hour delivery of complex, AR coated lenses, with its revolutionary Matrix system. This system and the wafer products used in the process were widely commercialised, however a cost effective solution for polycarbonate was never realised limiting its full potential in the North American market.
SOLA successes also include:
- Launching the first successful office lens with a product called ACCESS. These products are now widely dispensed as a second pair and or solution for emerging presbyopes and every significant competitor offers something in this category.
- Being the first company to develop a wrap compensated lens for both plano sunlens and Rx prescription (Spazio) applications.
- Launching a revolutionary 16 Base lens series under the Enigma and Contour Optics brands to provide truly optimal viewing in all directions. This highly innovative product depended on successful frame partnerships since the eyewear required a frame/lens solution and was therefore a little pre-emptive in the marketplace.
- Developing and commercialising a range of dispensing tools to monitor head and eye tracker behaviour and lifestyle.
- Being a pioneer in fully exploiting the flexibility of freeform manufacture with a design calculation system capable of morphing designs on the fly according to each patient’s vision, frame selection, lifestyle and visual behaviour. This was a truly remarkable development in the context that in the years preceding this tool, every lens design needed to be handcrafted for each base/add combination in each material. Now a design can be automatically generated from the morphing engine in any material with a combination of design attributes in less than a minute to suit each and every individual’s own needs.
- Developing numerous in-house measurement systems and manufacturing technologies that gave it a competitive edge in the market. These included surface measuring devices, automated production solutions, compression molding techniques amongst others.
While SOLA had great success developing new products, some did not lead to market success. A commonly held view is that during the last 20+ years, SOLA achieved limited success when it tried to be a market leader, e.g.
- Goldfish contour optics achieved no cash return after a lot of expenditure and success solving major technical and manufacturing issues (brilliant work was done by technical and manufacturing personnel).
- Matrix consumed enormous resources but had limited market success. It was possibly a flawed concept as some lenses were too thick and there was insufficient market for “1 hour AR coated lenses”. Matrix was offered to Essilor who declined). It was championed by John Heine right to the end of his tenure.
- Teflon AR coating led the way but did not lead to significant incremental sales. It became the marketing flagship brand and product for the belated North American Rx lab acquisition program of 2004, but never reached the aspirational levels initially set.
SOLA’s failure to be a market leader has been attributed to:-
- a weakness in Sales and Marketing
- ineffective interaction between R&D, Manufacturing and Sales and Marketing
- R&D taking too long and spending too much money
- the initial development of a too narrow product range requiring Mandrake to make it a market success
- beyond SOLA’s R&D capability but the CEO wanted it and no-one in R&D would tell him that they weren’t up to it, or that it simply couldn’t be done
– probably all weaknesses since Noel Roscrow’s time.
Barry Packham:
Around 2004 at a G14 meeting in Europe Herbert Weiss made the very astute observation that in his long experience in SOLA, that SOLA did poorly whenever it tried to lead the market, and did very well when it acted as a follower.
On the other hand, Karen Roberts notes:
What is driving a lot of the CZV business today are the things implemented in SOLA’s time that are still competitively differentiated and are the foundations of a lot of incremental business, e.g.:-
There are not that many of them but they are absolutely key technology things that are critical to CZV’s business.
- Freeform capability
- The morphing engines
- The patents put in place in some of the critical technology areas
Sometimes SOLA was its own worst enemy when looking at the success or failure of new products – unfortunately and if it was said often enough people actually believed it so that for a long time people thought Percepta (the progressive that SOLA launched in all material on day one – thus it required enormous effort and cost to get it up was a dud but Percepta continued to have a US$35 million revenue p.a. with 70 – 75% margins for years and years and even though it was stable it made such a huge contribution to our business for so many years .... and it has been talked about it as if it was a complete failure. If you looked at it as a percentage of SOLA’s profit it was a huge product and it was competing in a much more complex progressive arena than when VIP and XL products were launched and people forget that the share is diluted across 40 progressives not 5. So we always got carried away a bit when judging what was a success or failure without looking at the sustained contribution over many years.
A FEW OBSERVATIONS ON NEW PRODUCT AND TECHNOLOGY DEVELOPMENT Matthew Cuthbertson
Follow the Leader
Elsewhere in this history update, there are several comments related to SOLA’s natural position as a “fast follower “(not a leader) for implementation of new technologies and new products. I agree with this notion in large measure, although let’s not forget that SOLA introduced multiple very significant technology changes internally. Witness open-and-shut casting, spin coating, UV curing, the ATOM polycarbonate process, mold slumping, laminating, various measurement systems (especially powermeters) and many more.
But a very different dynamic came into play with putting a new product in the hands of a customer. This all derived from SOLA’s place in the value chain and the deployment of its internal investments. Certainly a sustained investment in research and development brought forward many new ideas and opportunities, but ultimately the company did not have the capacity for corresponding investments especially in marketing and capital equipment. Therefore the innovation pipeline faced significant (but appropriate) constraints; but SOLA really struggled to choose only the best-of-the best new ideas and to resource them properly. It was repeatedly seduced by its “nothing is impossible” culture and regional structure, into trying a little bit of everything everywhere.
The only significant level of marketing resource in the company resided in SOLA USA, with a customer base revolving around independent wholesale laboratories and chain retailers. In both of these channels SOLA was some distance away from the dispensing event and the product selection. Nevertheless, the company still enjoyed very significant success with introduction of new lens designs, especially progressives. I would argue that, in terms of lens design, SOLA may have been a fast follower during part of its history – but quickly established a capability that was on a par with its major competitors; in both the design and manufacture of optical surfaces and the underlying vision science.
However a very different set of circumstances applied to the introduction of new materials and coatings, which had to be compatible with a very diverse range of secondary processes in the hands of customers. (Clearly the introduction of a new material is a far more manageable process when you have complete control of the process in your own Rx laboratories, and SOLA’s later move into the lab business gave it far greater ability to leverage its capability in materials development.)
Spectralite
This is why I have always regarded the introduction of Spectralite as a watershed moment for the company, and a monumental achievement. VIP Gold embodied a new design, a new hard coating and new proprietary lens material – not to mention a revolutionary manufacturing process, requiring only modest levels of new capital investment. Not bad for a fast follower!
Also while Spectralite represented a clearly differentiated combination of the material properties, it still occupied a place in the well-established category of “mid-index” materials. So perhaps Spectralite represented just the right balance between the familiar and the unfamiliar to allow the international organisation to get behind the idea and to create a genuine global product (and a $100m business).
Of course there were many anxious moments along the way, not the least of which being a major problem with coating failure soon after the USA launch. But during this period SOLA exhibited its legendary ability to fix customer problems on the run and never look back!
The Spectralite story also emphasises a few of SOLA’s vices as well as its virtues. In the inevitable race to launch a new product there is always a tendency to build in cost, through expensive new features or underdeveloped manufacturing processes. This was particularly true for the Spectralite (especially the stock lens) although a very fruitful cost-reduction program was implemented once the product group became established. Importantly, this lesson was carried through to the Finalite launch, when a very comprehensive cost down program was initiated immediately after the product release.
Perils and Polycarbonate
In the period around 2000, the R&D organisation was devoting enormous effort to two projects (i.e. poly Matrix and Goldfish) which required the molding and coating of very difficult lens geometries, in polycarbonate – and remember that SOLA had little experience with large scale manufacture of polycarbonate lenses or indeed with high volume anti-reflection coating.
In my view this scenario (and many other aspects of the Matrix project) highlights a significant flaw in SOLA’s governance processes i.e. the lack of a systematic approach to risk management. On reflection this is particularly surprising for a USA listed company, attempting to manage a complex technology development program and a diverse new product pipeline. And it was also inconsistent with the disciplined approach to new product development employed elsewhere in the business.
The Matrix project was born from the single idea of delivering AR coated lenses within one hour. This motion of one-hour turnaround was important in the USA chain retail sector, but it’s not clear that the dimensions of this opportunity (or non-opportunity) were ever clearly understood. The fact remains that the core concept of Matrix delivered no tangible product benefits to the customer. But somehow the project evolved from an interesting research idea to an ugly full product launch (and subsequent withdrawal) without a rigorous analysis of the enormous technical, marketing, commercial and reputational risks involved.
Of course a notable exception on value creation could have been the use of lamination as a manufacturing route to polarising progressives, but that sad story is documented elsewhere.
NIH (Not Invented Here)
A particularly positive aspect of SOLA’s technology organisation was its ability to bring in new ideas from outside, and to work very constructively with other companies; even ones that were much larger and more complex that SOLA. Important examples are relationships with PPG/Transitions, Akzo Nobel, du Pont, SDC Coatings and Leybold Balzers; not to mention a score of smaller technology companies, leading customers and specialty chemical manufacturers such as Shin Nakamura.
The “not invented here” syndrome which hampers the innovative aspirations of so many companies was almost completely absent from the SOLA culture. This was particularly evident in the strong working relationships between the international research centres. There was strong but friendly rivalry, but this was always secondary to getting the job done and finding the best available solution.
PROGRESSIVE LENSES - a great profit earner for SOLA – probably the key profit earner after ~1990
The progressive lens story is well told in Breaking the Mold, p135, beginning in mid 1970. For SOLA it only received serious emphasis in 1982. Initially SOLA, via the Pilkington Ophthalmic Division, tried to licence the technology from AO but this approach never went anywhere so progressives were developed in-house. It was a very successful combined US/AU project largely brought about by the technical/political skills of Ted Ellis and the mathematical skills of Eric Barkan and Michy Kris. This was coupled with successful marketing in the USA by Bernie Friewald using contour plots for the first time to show the difference between SOLA and Essilor lenses.




The Essilor Varilux lens was the first progressive lens of modern design. It was developed by Bernard Maitenaz, patented in 1953, and introduced by the Société des Lunetiers (that later became part of Essilor) in 1959.
Essilor made a big hit with Varilux. Nine other progressive lenses were on the market by the time SOLA launched VIP/Graduate (its first progressive – but many more were to follow as indicated below)
There is a relatively new website http://www.wernerkoeppen.com by Werner Koeppen (who worked for Rodenstock and then Essilor) that aims to capture the progressive story entitled Progressive Memories. Kevin O’Connor helped Werner write the more detailed view of SOLA’s contribution.
Kevin provides the following insight in an email to Bob Sothman in March 2011:
Werner’s motives are to tell the story of the period where the Progressive Lens finally achieved the breakthrough on the worldwide market and about the big companies achieving this fundamental change - particularly acknowledging the people who contributed to this remarkable piece of history. Werner says in his introduction of Progressive Memories that he is well aware that it is a personal document in which the memory of his own projects is most clear. So certainly the names of some people who made significant contributions are not mentioned. But he considers the site as a living document and invites anyone who has different or more information, to contribute to the website so that it may become a more representative and accurate document.
From my contacts over the last 11 years with those who were directly competing with Zeiss, Rodenstock and Essilor, I now better understand the high regard which they all had for SOLA and its people – the VIP/Graduate and XL products (particularly the high level of control of manufacturing variables), ECP’s confidence in product performance, marketing innovation and commitment to customer support (e.g. the hotline in SOLA USA).
SOLA was for some considerable time regarded by our main competition as the ones to beat in the battle for market dominance, and progressives were the battleground. We had no real idea then that our technology was ahead of our European competitors – and so far ahead. We can be justifiably proud of this fact.
The story here describes the close competition (especially Essilor vs. SOLA), and how Essilor then pulled ahead of SOLA to dominate our industry.
There are maybe some lessons here about how to manage a successful business.
SOLA and SOLA/AO Progressives
| Brand | Launch Year | New Lens Design |
|---|---|---|
| SOLA | 1984 | VIP/Graduate |
| SOLA | 1987 | XL |
| 1988 | Smartseg, Poly XL | |
| 1989 | 1.5 mineral XL | |
| 1990 | SolAspheric SF | |
| SOLA | 1991 | Spectralite ASL, VIP Gold |
| 1992 | 1.6 mineral XL | |
| SOLA | 1993 | Spectralite ASL FT, XL Gold |
| 1996 | Access | |
| SOLA | 1997 | Percepta (all materials), Finished Access |
| 1998 | Visuality, Matrix XL | |
| 1999 | Icon, Opti-D | |
| SOLA | 2000 | SOLAMax |
| SOLA | 2003 | SOLAOne |
| SOLA | 2003 | SOLAOne HD |
| SOLA | 2004 | SOLAOne Ego |
| SOLA | 2005 | SOLAOne Ego+ |
| SOLA | 2009 | SOLA Elan |
| AO | 1990 | Technica |
| AO | 1992 | AO Pro |
| AO | 1995 | AO Force 55 |
| AO | 1998 | AO Compact |
| AO | 2000 | AO b'Active |
| AO | 2003 | AO Pro Easy |
One lens where SOLA led the way that is not mentioned in Breaking the Mould is the Myopia Control (MC) lens.
Myopia affects over 1.6 billion people globally, with two thirds of those affected live in Asia. The report in Optometry & Vision Science, edition 76, June 1999 by Leung and Brown of the Department of Optometry at the Hong Kong Polytechnic University into the effects on the progression of Myopia in Hong Kong Chinese schoolchildren when wearing progressive lenses, prompted SOLA to design, manufacture and distribute the SOLA MC Myopia Control lens.
The SOLA MC Myopia Control lens was the world’s first progressive lens designed specifically for children and young adults to reduce the progression of myopia. Further details are in the SOLA literature and video.
Andy Griffiths added the following about Access (enhanced near design lenses):-
The first in the field was Essilor with their Delta Horizon lens.
Their lens seemed overly complex to dispense and it seemed that Essilor didn’t have much up-take. However, we liked the concept. We wondered if we could make a product that delivered the better visual performance but without the dispensing complexities.
15 years ago there was still some nervousness about fitting PALs and we thought that maybe we could design a lens that was as simple to fit as a single vision. We adopted the KISS principle (Keep It Simple, Silly)
There are 2 critical features that contribute to success or failure with PALs - fitting heights and monocular PD’s.
Michy Kris had already done a very small and random assessment on how accurately PDs were measured and found a large variation between practitioners both in technique and results obtained. Therefore given that PD measurements were inherently flawed could we make a lens that used only this measurement.
We decided to fit the lens like a single vision reader, i.e. on near PD and optical centre on datum.
I conducted an assessment of many hundreds of PAL and bifocal fitting details. From this we worked out that the average pupil position was between 4 and 5 mm above frame datum. By some quirk of rate this was also the distance above optical centre that you needed to place the pupil to optimize single vision lens performance to compensate for frame pantoscopic tilt. We now had a pretty good starting point for our lens fit position and the design was refined to suit this concept.
I conducted a wearer trial at both SIHRC and my optometric practice office.
Initially at SIHRC we were aiming to confirm we had a design that worked and we fitted a number of wearers with different design lenses to see if one performed better than another. Once we had settled on a design we then fitted a number of wearers who would normally wear single vision readers with Access lenses and asked them their reaction.
Pleasingly we had a very large acceptance rate and we knew we had a product. At this stage I am not really sure if we intended to market it for ‘desk’ use but it soon became apparent that Access would be most useful for workers who used VDU and read a lot.
We also marketed the product to optometrist who used it to help young wearers with ‘learning’ difficulties to manage their close vision better. To this end we sponsored the Australian College of Behavioural Optometrists (ACBO) to tie in with their work.
We made Access in two ranges and we made guidelines for dispensing. However, the Low shift has been the most popular design and we sell them on a ratio of about 2:1 in Australia.
Unfortunately Access has not been taken up with great gusto in other markets and there have been string of clones and imitators released from other suppliers.
I conducted some work where I fitted the Access the same as a PAL but there seemed no benefit from this added complexity and indeed I have only had a handful of cases where wearers were intolerant to the fitting as recommended.
Over the years we have seen an expansion of the Access range of lenses so now there are no scripts that can’t be made and it’s now available in CR39, polycarbonate and 1.67 using freeform surfacing.
Publications included:-
- A clinical wearer study of the SOLA Access lens"", Clinical and Experimental Optomtery, March-April 1996, Anthony Hanks, Michy Kris, Leo Hartley, Graham Peachey, Anthony Simon
- Vertical placement as a determinant of acceptance for ACCESS near vision lenses"", Clinical and Experimental Optometry, Sept-Oct 1996, Michy Kris, Andrew Griffiths, Saulius Varnas, Scott Fisher
- Effect of small focal errors on Vision"", Optometry and Vision Science, July 1997, Anthony Miller, Michy Kris, Andrew Griffith
SOLA MATERIALS R&D FOR SPECTACLE LENSES AND COATINGS FROM 1980 – 2000 Huan Toh
During the post Second World War period up to the 1970s, spectacle or ophthalmic lens materials were made mainly from either mineral glass, or CR-39, allyl diethyleneglycol carbonate, a plastic thermoset material patented by PPG Industries of the USA. Compared to mineral glass CR-39 had the advantages of light weight, impact resistance, good optics and tintability, but was low in refractive index resulting in thicker edges especially for minus lenses worn by myopic or short-sighted users. In Japan where more than 90% of the wearers were myopic, a serious effort began in the early 1980s to develop an alternate higher index plastic material to reduce lens thickness for spectacle lenses. This effort was lacking in the USA and Europe because of the relatively low incidence of myopia, and the European perception that mineral glass is more stable and superior optically. In Japan, however, simultaneous AR (anti-reflective) coating development for spectacle lenses also began in earnest, because higher refraction of the lens results in an unacceptable high level of reflection, affecting both clarity of vision and wearer appearance. This represented a real challenge for SOLA in the 1980s, especially for the Ophthalmic Materials group in R&D.
Japanese lens companies were the leaders in high index lens and coatings development in the 1980’s. Hoya and Seiko released products like HiLux and HiLord, but they were ignored by USA and European markets because these first generation products lacked impact performance, tended to yellow and were poor in optical performance as measured by the chromatic aberration (Abbe number). SOLA started an ophthalmic materials R&D group from 1983, led by Huan Toh, to look into the development of a thinner lens materials which must also possess good optical, mechanical and process advantages over the prevalent Japanese materials. Using both polymer chemistry knowledge and structure-property modelling methodologies, a number of totally revolutionary approaches were taken to identify formulations which can deliver a “perfect” lens. It was very quickly agreed that one key strategy to deliver such a lens products was to identify and collaborate with a monomer, or “building block”, supplier. Hence began a global search, and a number of approaches and visitations were made initially by Ted Ellis (General Manager Technical) and Huan Toh, to identify potential partners. It soon became obvious that medium size specialty chemicals suppliers in Japan were the most responsive and cooperative for a new “unproven” business venture. Shin-Nakamura and Kyoeisha were two such companies who were prepared to provide and modify monomer and other molecular building blocks to SOLA for experimentation with no formal contracts or agreements.
SOLA Spectralite and Finalite

SOLA successfully obtained two key material patents in the mid 1980s which enabled it to deliver a “thinner and lighter” lens material with balance of optical, mechanical and process performances. This material was given the trademark “Spectralite” and had the additional advantage of being UV curable which resulted in a cure cycle of only a few minutes compared to 21 hours for CR-39. Spectralite was especially successful in the USA market because of its ease of surfacing, tinting and coating in the laboratories, compared to the Japanese high index lenses. Spectralite products enjoyed high sales for a period in the late 80s and early 90s, reaching an annual sales figure in excess of US$100 million.
In the 1990s Japanese material suppliers like Mitsui Chemicals intensified their R&D on high index ophthalmic lens material precursor development, and this resulted in a series of high and ultra index materials (1.60, 1.66 and 1.7+) with improved mechanical performance and color. SOLA responded with “Finalite” a 1.60 index material with good Abbe number, but it became obvious that it was getting increasingly difficult to compete in this field without the synthetic chemistry capability to invent and produce new material precursors. SOLA then adopted a new strategy by collaborating with USA chemical company PPG who had chemical synthesis capability. However this effort was too little and too late, as it could only deliver a 1.60 material called “SOPP” with the only advantage of being cheaper than the prevalent Mitsui material MR-8. This project was soon abandoned.

Photochromic


One other effort being pursued during this period by SOLA was the development of “Velocity”, a lens substrate compatible with the Transitions photochromic imbibation process, which delivers the fastest fade-back speed on the market.
This was achieved through the clever manipulation of the Spectralite material chemistry with the Transitions imbibation process. Again the lack of chemical synthetic capability in developing new dyes limited this development.
Coatings
For many years SOLA depended primarily on Japanese company NSG (Nippon Sheet Glass) who supplied a hard coating code named PermaGard for SOLA’s CR-39 based products. SOLA R&D, primarily in the USA (Petaluma), focused its development on coating process application technology, especially spin coating. It was only in the 1990s that an effort began in Lonsdale to look at sol-gel technology development to deliver coatings with value-adding features including high index coatings.
Nanotechnology
In the Carl Zeiss Vision era, there was a change in R&D strategy, where external companies with technologies compatible with internal needs were identified and pursued for partnership. One such thrust was in the area of nanotechnology to provide super hard and other functional coatings (anti-fog, photochromic, easy clean etc).
THE STORY OF PHOTOCHROMIC PLASTIC LENSES Bob Sothman
Almost from the day photochromic glass lenses were first introduced in the 1960s, plastic lens manufacturers received requests for a photochromic lens made from light-weight plastic.
PPG began work on plastic photochromics in 1973. In 1983, PPG made two breakthroughs with the discovery of a new family of photochromics called pyridobenzoxaines and the development of a unique imbibation process for incorporating photochromic properties in plastics. (Note that in the early 1980s Plessey was using an imbibation process – refer 2.5 next section.) From then until Transitions Optical was formed in 1990 by a joint venture between PPG and Essilor, PPG spent US$1 million on testing the technical and marketing feasibility of plastic photochromic lenses.
By contrast, SOLA started working in the late 1970s on developing a photochromic plastic lens. SOLA was experimenting with photochromic materials developed by the Royal Institution in London (the place where Michael Faraday did much of his groundbreaking research on electricity). After Pilkington purchased SOLA, the development accelerated as Pilkington and their collaborators developed new types of dark-coloring photochromic dyes and techniques to cast these directly into spectacle lenses. Good progress was made and the first product to be commercialised was a blue photochromic plano lens to be cast in SOLA, Italy in 1988 i.e. several years before Transitions had any saleable product in 1991. SOLA could cast the product with good yields in SOLA Italy but the lens had a "hoop stress" at the edge when viewed under polarising light. The area would have been edged off when fitted to virtually all frames so was not really a problem but the SOLA Sunlens marketing folk said they could not sell the lens with such an imperfection and the product was never marketed.
Despite lack of success implementing the “blue” photochromic sunlens, SOLA and Pilkington continued R&D work on plastic photochromics through the early 1990s, until the time of the sale of SOLA to AEA. During this period, the chemistry of the photochromic dyes was refined to produce compounds which colored to strong neutral hues in sunlight. Consequently, new casting techniques were developed to incorporate the dyes into ophthalmic rather than plano lenses, focussing on “Spectrum” materials and SOLA’s proprietary UV-curing technology.
The outcome of this initiative was a laboratory-scale casting process to make lenses with the following features:-
- Based on SOLA’s Spectralite mid-index lens material
- Semi-finished blanks, which could be produced in single vision, bifocal or progressive form
- Compatible with SOLA’s existing mass-production casting and hard coating processes
- Photochromic properties which were competitive or superior to the Transitions Plus lenses released into the market around the same time
Although in-house plastic photochromic lenses were never commercialised by SOLA/Pilkington, SOLA did accumulate a valuable portfolio of Intellectual Property over the course of the development program and several key patents have subsequently formed the basis of competitors’ ongoing product evolution. With the split from Pilkington, much of the IP of lens applications was licensed or sold to third parties.
In 1995, negotiations commenced between PPG, Transitions and SOLA management regarding business collaboration. The business proposal was based on SOLA selling and/or licensing its technology and patent know-how to Transitions for an up-front fee and ongoing royalty stream. Concurrently SOLA would then use Transitions photochromic product exclusively so that through their combined marketing and sales efforts, they would build the plastic photochromic category as a competitive alternative to glass photochromics. SOLA, PPG and Transitions believed this combination was the best way to create demand for a photochromic alternative which did not then exist.
In February 1996, SOLA and Transitions entered into a 10 year Agreement which formalized the collaboration. SOLA sold Transitions its photochromic technology know-how, granted licenses to select SOLA patents and sub-licenses to select Pilkington patents.
Transitions Optical generated sales of:-
- ~US$50 million within 3 years
- ~US$300 million by 1999
- US$700 – 800 million currently
At the time Transitions comfort lenses were first introduced, the lens market in the USA was ~80% plastic and 20% glass. Half of all glass lenses were photochromic. Rather than focussing on those patients who had been wearing heavy photochromic glass (10% of the market), Transitions focussed on the consumers who already wore plastic lenses (80% of the market) and on those who wore fixed tint (60% of the market). This approach, along with opening up a completely new premium lens market, was an enormous success. Transitions lenses turned out to be the most successful and fastest segment of the entire premium lens field, all made possible by proper positioning of a brand new technology.
** includes information from PPG publication CR-39 - CELEBRATING 50 YEARS
THE SOLA PHOTOCHROMIC STORY Ian Threlfall
OVERVIEW
Long before the launch of the first widely marketed plastic photochromic lens by Transitions in 1991 and the subsequent technical initiatives with Spectralite Transitions and Velocity, SOLA and Pilkington carried out a substantial programme of work through the 1980s and into the 1990s to develop all the fundamental components of a viable photochromic lens.
The inception of the work was at Pilkington in the late 1970s. It became a joint collaboration with SOLA R&D and several UK universities from the mid-1980s and, after several major changes of scope, concluded in 1993 when Pilkington sold its vision care business to AEA. Much of SOLA’s photochromic technology and intellectual property was then sold or licensed to PPG and Transitions in the mid-1990s and was incorporated into subsequent generations of Transitions products.
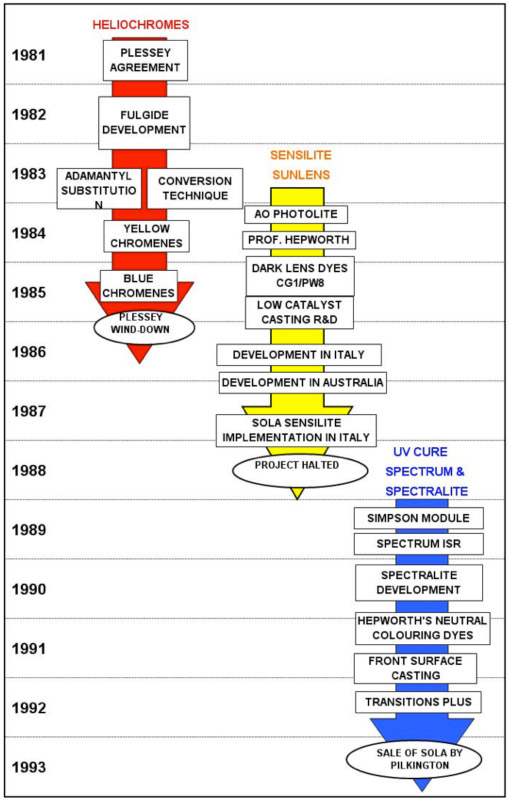
The evolution of the technology occurred in three phases:-
- Heliochromes and the CR-39 Reactolite look-a-like (1980 – 1985)
- Dark Lens compounds and the SOLA Sensilite sunlens (1984 – 1988)
- Neutral colouring ophthalmic lenses in Spectralite (1988 – 1993)
Throughout its development, the program was concerned with four main challenges:-
- Identifying and manufacturing photochromic dyestuffs with the right balance of sunlight sensitivity and darkening and fading properties to make a saleable lens.
- Developing a polymeric substrate which could successfully incorporate the dyes and was also a viable ophthalmic lens material.
- Chemically stabilising the dyes within the lens so that photochromic activity is retained over the life of the patient’s spectacles – the so-called fatigue problem.
- Developing a manufacturing process which was as close as possible to the processes used for conventional non-photochromic lenses, and which had an economic cost structure.
Although SOLA never commercialised a plastic photochromic lens of its own, the various development programs did generate products which were at the forefront of photochromic technology at the time. The program gave rise to a number of genuine inventive steps and key patents covering the chemistry of photochromic dyes and their incorporation into plastic lenses.
PHOTOCHROMIC DEVELOPMENT TIMELINE
1. Technical background: How Photochromic Lenses Work
Unlike the silver halide-based chemistry which drives glass lenses, plastic photochromics usually depend on the use of sunlight sensitive organic dyes which are dispersed into the lens polymer. The dye molecules are changed from colourless to a coloured form by the UV energy present in sunlight. The coloured form reverts back to the original colourless form when the activating light is removed. This darkening and fading process must be repeatable time and time again, each time the wearer goes outdoors into the sun or returns to the shade.
To be competitive with glass lenses and have broad market appeal, a plastic photochromic lens must darken to a stable neutral colour such as grey or brown, and should not deviate significantly from neutral hues during the darkening and fading cycles. As with textile dyes and most printing inks, organic photochromic dyes tend to exhibit specific colours rather than greys or browns. This means mixtures of two, three or four different colouring compounds will be needed to achieve neutral hues, which rely on a broad absorption across the full visible spectrum.
In addition to the fundamental requirement for neutral colours, the lens must also meet a long list of other criteria, including:-
- Darkening speed (not too fast and not too slow).
- Fade-back speed (not too slow),
- Transmission in the darkened state (not too dark, not too light),
- Temperature sensitivity (minimum sensitivity to variation in ambient temperature)
- Minimal sensitivity to reversal by visible light.
- Resistance to degradation over multiple darkening & fading cycles.
Organic molecules with the right balance of photochromic properties do not occur in nature and are not available from commercial sources. Anyone undertaking to develop a plastic photochromic product will need to firstly identify the structures of organic molecules which are likely to have the right balance of properties, then synthesise and purify these compounds in the laboratory and finally evaluate the actual optical performance of the dyes when incorporated into a plastic lens. The chemical structures of the dyes can become quite complicated, which usually means multiple step synthetic and purification procedures and needs considerable chemical skill and experience to produce the molecules from readily available starting materials.
Furthermore, the polymer matrix into which the dyes are incorporated must be a viable material for use as a spectacle lens. The polymer must meet both ophthalmic industry expectations and mandatory national and international performance standards. The lens must also be capable of being manufactured consistently and at a cost that makes the final product economic for the manufacturer.
2. HELIOCHROMES AND THE CR39 REACTOLITE LOOK-A-LIKE
2.1 Commercial Foundation: Threat to Reactolite Rapide
In the late 1970s, Pilkington’s traditional markets for glass spectacle lens blanks were threatened by the growth of CR-39 – a concern which eventually led to the purchase of SOLA. Pilkington were also aware of the danger to their lucrative glass photochromic lens business if a competitor released a plastic alternative.
Although there were no plastic photochromic lenses on the market at the start of the 1980s, it was clear from scientific and patent literature that competitors were working towards this objective. At that time Pilkington had just developed and launched Reactolite Rapide, their second generation glass photochromic product, and the future revenue stream from Reactolite was clearly at risk from plastic.
It was also clear that most of the technologies necessary to develop and manufacture plastic photochromic lenses were well outside Pilkington’s core glass-making expertise.
2.2 The Battelle Report
To properly evaluate their options, Pilkington’s Ophthalmic Division commissioned Battelle International, a global technology & innovation company, to carry out a survey of the status of organic photochromic systems worldwide. This initiative got underway around the time the purchase of SOLA was being finalised and Pilkington’s first entry into the CR-39 lens business. Battelle were to review classes of photochromic materials with potential applicability to ophthalmics and identify organisations which might be available to collaborate with Pilkington to develop a product.
The Battelle Report identified the most promising option as Professor Harry Heller, based at the Chemistry Department of the University of Wales in Aberystwyth. Heller was an expert on fulgides, a family of heterocyclic organic compounds which were known to have interesting photo-active properties and had formed a spin-off company (Aberchromics Ltd) to pursue commercial exploitation of these materials.
At the time of the Battelle Report, no competitor had filed patent claims for fulgides for spectacle lens use, and prior to Pilkington’s approach, Heller had not completed any detailed investigation for this application either. This meant Aberchromics did not have compounds available immediately “off the shelf” for testing. However the fulgide family did appear to be a promising source of new photochromic molecules which could be tailored to the needs of plastic spectacle lenses, and for which Pilkington could control the intellectual property.
2.3 The Plessey Agreement
Despite having promising technology, there was a hindrance to Pilkington having direct access to Professor Heller and Aberchromics. Aberchromics was already under contract to Plessey, a large UK electronics company, to develop fulgides for electro-optical applications.
Plessey was mainly interested in solid-state data storage devices and displays, which required photochromic dyes with quite different properties to those needed for spectacle lenses. For data storage, the compounds did not necessarily have to absorb visible light. The photochromics could be switched between their two optical states by two lasers emitting light at different wavelengths, one or both of which could be operating in the infra-red part of the spectrum. Once switched into a particular state, the fulgides were required to stay in that state until switched back by the second laser. However the terms of the existing Plessey – Aberchromics collaboration precluded Heller from dealing independently with Pilkington in the ophthalmic area.
The contractual issue was overcome by Pilkington entering into a joint development agreement with Plessey, who then sub-contracted Heller and Aberchromics to synthesise the new compounds. The contract was negotiated such that Plessey also had a technical role in the program – for which Pilkington were required to pay.
The project was structured with the following roles:-
- Aberchromics (University of Wales): Synthesis and supply of new sunlight-activated photochromic fulgides for evaluation by Plessey & Pilkington.
- Plessey (Caswell Research Centre): Molecular modelling to identify structural characteristics which would make the fulgides suitable for the spectacle lens application. Plessey were also to investigate techniques to incorporate Heller’s compounds into ophthalmic materials.
- Pilkington: (Lathom Research Centre): Assessment of plastic photochromic samples using specialised photochromic measurement equipment developed during the Reactolite programs.
In effect, Plessey were to develop the fundamental photochromic lens technology for Pilkington, Pilkington would decide when the performance was adequate for ophthalmic use and eventually transfer to SOLA for detailed product development, scale-up and commercialisation.
2.4 Heller’s Heliochromes
Starting around 1981, the first objective of the Plessey/Aberchromics/Pilkington collaboration was to develop a family of fulgides which would darken in sunlight to a range of primary colours, covering the visible spectrum from purple and blue to orange or yellow, and which could be successfully incorporated into CR-39 to produce a stock lens with optical properties similar to commercial glass lenses like Reactolite Rapide or Corning’s PhotoGray Extra.
The starting points for Aberchromics were several relatively simple photoactive fulgides developed for Plessey, and progressed to more elaborate compounds as the influences of molecular structure on photochromic performance were better understood and quantified. The process was iterative and involved Heller’s chemists at Aberchromics synthesising and purifying small quantities of candidate fulgides and provided samples to Plessey and Pilkington for assessment.
When the project had proceeded to the stage where useful sunlight-sensitive fulgides became available, Heller coined the term Heliochrome, to distinguish sunlight sensitive compound from those which operated in the far UV or infra red parts of the spectrum. The Heliochrome description was widely used within the project and in Aberchromics patent claims, but unfortunately has not persisted.
After a period of systematic experimentation, Aberchromics produced several fulgides which could be activated by the near UV part of the solar spectrum, and which coloured to suitable blue or purple colours. The problem with these compounds was that the fade rates of the coloured forms were very rapid at normal temperatures. The consequence was a lens could not darken to a suitable transmission, unless the wearer was using the product in sub-zero conditions. The challenge was how to slow the fade rate of the Heliochromes to make them usable on a normal sunny day, whilst maintaining their sensitivity to solar UV and their acceptable darkened state colours.
2.5 The Incorporation Problem
Under the Plessey Agreement, while Heller and the Aberchromics group were working to synthesise new fulgides, a separate group within Plessey’s Research Centre at Caswell in Northamptonshire started work on ways to incorporate them into suitable plastic materials.
Since Pilkington’s initial objective was a CR-39 lens with Reactolite photochromic performance, CR-39 was the material of primary interest, although other plastics which might have some application across Pilkington’s diversified business were also considered. These ancillary polymers included injection molded PMMA, Polycarbonate and Cellulose Acetate Butyrate.
In reality, Pilkington researchers at Lathom carried out most of the incorporation studies involving CR-39. This was due to Pilkington having access to CR-39 monomer and initiators, together with molds, gaskets and cure profiles from SOLA UK in Birmingham. SOLA UK was involved in a small-scale casting operation at the time and having direct access to SOLA’s raw materials and proprietary casting equipment gave Pilkington R&D much more flexibility for experimentation.
The simplest way to incorporate fulgides into a CR-39 was to dissolve the dyes in monomer, then cure the lens as normal. Unfortunately the molecular structure of the fulgides was such that they were chemically attacked by the high concentrations of active free radicals generated during CR-39 polymerisation process. The result was a cured lens with no remaining photochromic properties and it appeared fulgides were fundamentally unsuitable for direct casting into CR-39.
An alternative incorporation technique developed at Plessey was high temperature imbibition. This was analogous to the conventional tinting process used in the ophthalmic industry, but used a dispersion of photochromic compound in an inert silicone oil and a temperature around 120 or 130 °C. Both CR-39 polymer and the fulgide dyes had sufficient thermal stability to undergo high temperature imbibition without major degradation and the result was a CR-39 lens which could be activated in sunlight. However the amount of photochromic compound transferred to the lens during this process was quite low, meaning the lens did not go sufficiently dark, and was prone to very rapid fatigue, meaning photochromic activity was lost after a relatively small number of cycles. The outlook was therefore that fulgides could not be imbibed into CR-39 to give a lens with saleable photochromic performance.
2.6 Fulgimide Yellows
Most of the early sunlight-sensitive fulgides were activated to blue, purple or red colours. To make a Reactolite-like plastic lens, dyes were also needed in complementary colours (yellow and orange) which would allow grey or brown-colouring lenses to be made.
The most promising molecular structures which appeared likely to yield yellow or orange compounds were the fulgimides, a variation of the fulgide family. However all the yellow and orange-colouring fulgimide structures investigated by Aberchromics had some significant problem which prevented their use – either the compounds were very difficult to synthesise or purify, or once made they were unstable and unlikely to have a useful fatigue lifetime in a lens.
2.7 Three Key Inventions from the Plessey Collaboration:
After several years of work, by early 1983 the Plessey / Aberchromics/Pilkington collaboration had developed novel blue and purple-colouring fulgides which could be activated by sunlight. However there were a number of roadblocks preventing further progress:
- The fulgides did not go dark enough at ambient temperatures to produce a saleable lens (due to fast fade-back).
- Unable to incorporate enough fulgide into the lens to give acceptable performance – in particular the fulgides were totally destroyed by CR-39 curing conditions.
- Blue and purple colours were achievable, but fulgides with complimentary yellow and orange colours were proving elusive and may not be achievable.
These three issues were overcome by separate inventions and were all subsequently patented.
a) Adamantyl group substitution
This invention by Aberchromics was the key to making fulgides with the right darkening and fading speeds for ophthalmic use, and lenses which are capable of matching the transmission of photochromic glass at ambient temperatures.
The effect was patented (GB 2146327 Heller et al)
The invention depends on the steric hindrance caused by introducing an adamantyl group at the key “ring opening point” of the fulgide molecule. The adamantyl group is a bulky, rigid, chemically inert hydrocarbon ring system, whose effect is to slow the fade-back rate without any adverse effect on other photochemical properties of the fulgide.
b) The Conversion Technique
This invention provided a way to successfully cast fulgides directly into CR-39 lenses.
The technique was jointly patented by Pilkington, Plessey and Aberchromics (US 4576766 Baskerville, Maltman & Oliver)
As mentioned in 2.5, fulgides could not be cast directly into CR-39 monomer because of total degradation of the photochromic properties by chemical species generated during the cure. Fulgides could be incorporated into CR-39 lenses by imbibation, but the quantity of dye transferred was low, the darkening potential of the lens was inadequate and the fatigue rate was very rapid.
The chemical synthesis of heliochromic fulgides was generally a laborious and time consuming process. To get from simple, commercially available starting materials to the final photochromic molecule involved a number of steps and isolation and purification of distinct intermediate compounds. The last step in the synthetic sequence was an internal ring re-arrangement of the so-called fulgide precursor to generate the final photo-sensitive molecule. This molecular re-arrangement of the non-photochromic precursor was achieved by either heating the precursor in a solvent overnight or alternatively by exposing a solution to a UV light (a UV black light at 365nm was effective, but sunlight was slow and not very effective due to the low intensity of suitable wavelengths).
During mid-1983 it was realised that the thermal conditions used in the laboratory for the precursor-to-fulgide conversion were very similar to the thermal conditions used to cure CR-39 lenses. Furthermore, the chemical backbone of the precursor molecule was different to the structure of the final fulgide and may be less sensitive to attack by free radicals during the polymerisation of CR-39.
It was found that by dissolving the precursor compound into CR-39 monomer and using a normal cure process with IPP initiator, enough precursor would survive the cure to be subsequently converted to fulgide and achieve a useful colouring photochromic lens. In effect, the last step of the fulgide synthesis was being carried out in situ within the CR-39 lens.
The temperature regime of an 18 hour CR-39 cure cycle was enough to convert some of the precursor to fulgide, and the lenses would be weakly photochromic as soon as they were removed from the molds and cooled. To convert most of the precursor to fulgide and achieve a strong photochromic response, the cured lens was exposed to a low intensity UV lamp for around 16 hours, after which most of its photochromic effect was developed.
Because the photochromic fulgide was dispersed in bulk throughout the CR-39 polymer, both the darkening potential of the lens and the fatigue life were greatly improved compared to imbibation. It was believed that a reservoir of unconverted precursor would remain in the lens which would be slowly converted to fulgide by sunlight during normal wear and would provide a reservoir of new active photochromic molecules to offset fatigue. This was confirmed by measurements which showed lenses made by this “Conversion Technique” would show slightly improved photochromic response after an initial period of use compared to when freshly manufactured.
c) Yellow Chromenes
This invention led to a new family of photochromic compounds which were beyond the scope of the original Plessey agreement and gave access to a series of yellow colouring photochromics needed to generate a neutral colouring lens. This approach was patented in US 4818096 (Heller et al)
2.8 Final Outcomes of the Plessey – Heller - Pilkington Collaboration
By late 1984 and early 1985, the three-way program had developed and patented:-
- Purple and blue-colouring fulgides with reasonable performance in sunlight
- Yellow colouring chromenes with reasonable performance in normal sunlight.
- Laboratory-scale techniques to incorporate the new dyes into CR-39 lenses.
On a laboratory scale, photochromic lenses could be produced with the following characteristics:
| Lens Material | CR-39 |
| Curing system | Thermal cure – similar to normal CR-39 stock lenses |
| Initiator system | IPP/SIP – concentration reduced by 15 – 20% |
| Photochromic system Dual system: | fulgides + chromene |
| Bleached state colour | Pale yellow background colour |
| Darkened state colour | Grey or brown colours achievable. Similar to Reactolite Rapide or Photogray |
| Darkening performance | Darkening rate generally good. Lens shows initial purple colour bias when darkening |
| Fade-back performance | Fade rate faster for purple component than yellow component. Result: yellow bias during fade |
| Temperature sensitivity | Poor. Colour mismatch at high & lower temperatures. Purple lens at low temp, yellow lens at higher temps. |
| Fatigue resistance | Moderate for fulgide – probably not adequate. Better for chromenes – but yellow fatigue products |
Advantages
- Cast CR-39 material.
- Stock lens range seemed plausible.
- Activated transmission similar to transmissions achieved with photochromic glass under laboratory test conditions and in the UK sun.
- Neutral gray or brown coloured activated state under laboratory test conditions and in the UK sun
- Potentially the first neutral-colouring plastic photochromic product into the market.
Disadvantages
- Darkening & fading rates of fulgide and chromene dyes were not well matched. While a neutral colour could be achieved in the stable darkened state, the lens went through purple colours while darkening and stayed yellow during fading.
- The temperature sensitivities of the two photochromics were not well matched, meaning non-neutral colours developed in hot or cold weather conditions.
- The fatigue resistance of the fulgide was inadequate, resulting in a lens which developed a colour bias towards yellow after a limited period of use.
- Manufacturing complexity. Although the cure process was similar to that used for conventional CR-39 lenses, several significant process steps would need to be added for the photochromic lens and new equipment would need to be developed for these. For example mixing the fulgide precursor into CR-39 monomer, conversion of the precursor to the fulgide with UV lamps and high temperature imbibation baths to incorporate the yellow colouring chromene into the lenses. The extra manufacturing complications, labour costs and yield losses may well have made the photochromic CR-39 product uneconomic to manufacture.
- Expensive photochromic dyes. Although no serious scale-up studies were undertaken for commercial quantities, the heliochromic fulgides were likely to be expensive to manufacture because the required molecules involve difficult, multi-step syntheses with some low yielding stages.
The fulgide precursors need to be used at relatively high concentrations for the cast CR-39 application, due to some sacrificial loss during the cure.
The chromene series of dyes can be manufactured more easily and cheaply but would be used inefficiently in the high temperature imbibation baths.
2.9 People involved in the Plessey – Heller - Pilkington Collaboration
- Pilkington*:
- Bill Maltman
- Mary Ormsby
- Ian Threlfall
- Aberchromics **
- Harry Heller
- Clive Trundle
- John Whittal
- Steve Oliver
- Plessey **
- Jack Brettle
- Martin Baskerville
* In addition to the core R&D team the hierarchical management structure within Pilkington meant several tiers of management were regularly involved in the plastic photochromic project and especially management of the Plessey collaboration.
These include John Bradshaw, Gordon Loukes, Don Wright, Jim Procter and Harold Charnock. Tom Jackson from the Pilkington patent department was heavily involved in the Plessey contract and IP aspects of the project.
** Other workers were involved in the early stages of the collaboration at both Plessey and Aberchromics. Those indicated above were involved in the later stages, leading to lenses which showed useful photochromic properties.
3. DARK LENS COMPOUNDS AND SOLA SENSILITE
1984 was an important year in the photochromic project. This year saw the appearance of a number of new personalities on the Pilkington and SOLA scene and other key developments such as Project Leapfrog and the release of AO Photolite. These factors combined to re-direct the photochromic program away from the original CR-39/Reactolite objectives and reliance on Harry Heller, into directions more amenable to early commercial exploitation.
3.1 New People & Personalities:
- Tony Ledwith. Formerly Professor of Industrial Chemistry at Liverpool University, Tony Ledwith joined Pilkington as Deputy Director of Research in 1984 and took a strong interest in the plastic photochromic program from the start. Ledwith was not too tolerant of the rate of progress being made under the Plessey Agreement and as a senior academic he had sufficient gravitas to match Heller during professor to professor discussions on technical matters. Ledwith also believed that ancillary work, such as development of incorporation techniques and fatigue studies, which had been outsourced to Plessey as part of the collaboration agreement could be better carried out internally at Pilkington.
- Ted Ellis. In 1984 Ted Ellis returned to the UK from a period of secondment at SOLA in Australia and took up a position as Technical Director of Pilkington’s Ophthalmic division. Prior to SOLA, Ellis had a strong background in glass photochromics and was now able to facilitate better links between the R&D group at Lathom and various parts of the SOLA technical organisation. This would include stronger links with SOLA R&D in Lonsdale and linkage to SOLA ADC in Ireland and SOLMA in Italy.
- Colin Perrott: With Ted Ellis’s return to Pilkington, Colin Perrott joined SOLA as Group Technical Manager in 1984. Perrott was instrumental in progressing Project Leapfrog and in moving SOLA’s new products strategy away from simply extensions to the CR39 product range and towards more step-out lens materials and technologies.
- Huan Toh: Huan Toh joined SOLA in Australia in 1984 and immediately became a useful source of monomers and industry contacts, which would subsequently allow the development of new lens materials. The properties and curing of these new polymers could be tailored to be much more amenable to photochromic dyes and the general needs of photochromic systems than is the case with CR-39.
- John Hepworth: John Hepworth was Professor of Chemistry at Preston Polytechnic (now known as the University of Central Lancashire). He led a small group of post-docs at Preston who were working on novel organic compounds of interest to the local colour chemistry industry. Although not directed specifically at photochromism, the compounds which Hepworth and team were developing were structurally similar to the photochromic chromenes.
Hepworth had not been identified by the Battelle Report (since he was not working on photochromic materials at the time) and the link was discovered accidentally during a casual literature search at Pilkington on the chemistry of chromenes. Unlike Heller, Hepworth was not encumbered by any restrictive agreements with third parties and was free to work under contract to Pilkington. Hepworth’s group was also conveniently located, only 35 km from the Lathom R&D Centre. - Martin Rickwood: Rickwood was a synthetic organic chemist recruited by Pilkington in late 1984 as part of an initiative to set up an organic synthesis capability at Pilkington’s Lathom R&D centre. Rickwood was completing a PhD at the University of Edinburgh when recruited and subsequently worked with both the Heller and Hepworth groups to continue development of new photochromic compounds.
- George Tennant: George Tennant was Professor of Chemistry at the University of Edinburgh and had been Martin Rickwood’s PhD supervisor. Prof. Tennant was employed as a consultant to Pilkington for several years during the mid-1980s while Rickwood was setting up the in-house synthetic capability at Lathom. Tennant was employed in parallel areas of investigation to Hepworth and the Preston group.
3.2 New Direction - Project Leapfrog:
Leapfrog was an initiative emerging during 1984 as a crystallisation of SOLA’s earlier attempts to pursue improved methods of delivering plastic spectacle lenses beyond the capabilities of conventional CR-39 casting.
While the two main outcomes of the project have been the Spectralite lens material and the ATOM process for polycarbonate, Leapfrog also provided a significant change in direction for the organic photochromic program by encouraging investigation of alternative lens materials and legitimising a move away from the focus on the CR-39 Reactolite look-a-like.
Around the time of Leapfrog’s inception, the Pilkington technical team at Lathom were starting to look in more detail at alternatives to Heller’s heliochromes. This was partly prompted by the release of American Optical’s AO Photolite lens onto the market and was facilitated by a sample of a new photochromic dye obtained by chance from a Pilkington glass lens factory in France. Subsequently a wide range of new proprietary photochromics was produced, independently to Heller and Plessey.
From 1985 onwards, the new dyes developed by Pilkington and its collaborators were almost exclusively incorporated into the “Spectrum” type polymers emerging from Leapfrog. While not always 100% ideal, the chemistries of Spectrum materials and their curing processes could be manipulated to be amenable to photochromic dye incorporation – an option which was not easily accessible with CR-39 or injection molding. By 1986, work towards CR39 photochromic lenses based on Heller’s Heliochromes had effectively ceased at Pilkington.
3.3 AO Photolite appears
AO Photolite was the first plastic photochromic lens to be commercially available and had a limited market release around 1983 - 1984. The lens was based on a CR-39 substrate and a blue colouring spiro-oxazine (SINO) dye. The lens transitioned from almost colourless in the unactivated state to pale blue in sunlight.
Samples of the AO Photolite lens were obtained and assessed by Pilkington under standard photochromic test conditions. The evaluation concluded that while the speeds of darkening and fading were comparable to glass lenses, the darkened state transmission was too pale and the blue colour too far from neutral to be a strong competitor to the major glass photochromic products. It was believed from patent literature that AO manufactured the lens by a high temperature dyeing process, using a dispersion of the dyes in a glycol bath at a temperature somewhere above 100 °C.
3.4 The Vergo SINO dye
In 1981 Pilkington had purchased the former AO Vergo glass lens factory at Goetzenbruck in France. This facility was re-named Pilkington Optique SA (POSA) and subsequently became IOSSA under SOLA management. While Pilkington were acquainting themselves with the new purchase, a plastic bag was found in a cupboard at Goetzenbruck, containing a few hundred grams of a yellow powder. The bag was unlabelled apart from the word “Photolite” written in texta. The material eventually found its way to Pilkington’s Lathom R&D Centre, where a chemical analysis showed it to be a particular example of the SINO family of compounds claimed in American Optical’s patent US4215010 (Hovey, Chu et al.).
The SINO photochromics had not been proposed as an option by the Battelle Report, presumably because the relevant intellectual property was covered by AO, who would be unlikely to licence it to Pilkington.
Some small scale experimentation was performed on the dye from Goetzenbruck, using techniques developed during the heliochromes program. These tests showed the AO dye had the following characteristics:-
- The SINO could not be cast directly into CR-39. All of the active dye is destroyed during the cure and the resulting lens is non-photochromic.
- The SINO can be dyed into CR-39 using high temperature imbibation (from silicone oil in Pilkington’s case) and gave lenses with roughly the same photochromic properties as the commercial AO Photolite lens.
- Although the darkened state transmission was not considered adequate, the fatigue life of the lens was very good compared to fulgides and could possibly meet the benchmark for durability of a commercial product.
- The AO SINO could be successfully cast into polymers other than CR-39, provided small quantities of polymerisation initiator were used and highly active peroxy percarbonates like IPP were avoided. For example the SINO dye could be dissolved in methyl methacrylate monomer and cured to make PMMA samples which exhibited a very good balance of photochromic properties, better than anything achievable in CR39. However PMMA was not a viable ophthalmic lens material.
Having assessed the strengths and weaknesses of American Optical’s SINO dye and the AO Photolite lens and with the knowledge developed during the heliochromes program, the following questions were considered:-
- Can the molecular structure of the SINO be modified using some of the techniques developed for heliochromes to make a darker colouring dye which retained the good characteristics of the AO compound?
- Can the darker colouring SINO dye be incorporated by direct casting into the new types of lens materials being proposed within Project Leapfrog?
- Once incorporated into a lens, will these inherently more stable dyes have adequate fatigue life for a commercial lens?
3.5 The Dark Lens Compounds
In 1984 one of the first tasks which Pilkington assigned to Professor Hepworth and the team at Preston Polytechnic was to study ways to improve the darkening potential of photochromic SINOs. This outcome was achieved rapidly and Hepworth’s group provided a considerable number of new SINO dyes for assessment at Pilkington during 1985 and 1986.
The new compounds became know as “Dark Lens” materials because of their ability to colour to much higher densities than any previous photochromic dyes, even when used at relatively low dye concentrations. The structural modifications which Hepworth employed for Dark Lens SINOs were different to the strategies used by Heller and Aberchromics, and the invention was patented (US4913544, Rickwood & Hepworth).
There were some compromises to the good photochromic properties seen in the American Optical SINO from Vergo – for example the fade rate and temperature sensitivity of the Dark Lens materials was greater and the fatigue rate on a molecule-per-molecule basis was worse with the new compounds. However, the fact that dark-colouring lenses could be achieved with small concentrations of dyes outweighed these and none of the drawbacks were thought to make the product unsaleable.
Some of the Dark Lens photochromics produced by Hepworth were so fast to activate and capable of colouring to such extreme optical densities that they were evaluated in a separate program by Pilkington’s Electro-Optical Division and the UK Ministry of Defence for use in visors to protect air force pilots from being blinded by nuclear explosions. These particular SINOs were much too strongly colouring for spectacle lens use, and had poor fatigue resistance; however if a pilot gets to witness more than one or two atomic explosions, a fatigued photochromic visor is likely to be the least of his worries. It is not known whether these compounds proceeded beyond the evaluation stage into any military applications.
In fact some of the first compounds to be developed during Hepworth’s initiative proved to be the ones best suited to use in commercial spectacle lenses. The two SINOs selected for larger scale development were known as CG1 and PW8 (the initials indicating Chris Gabbutt and Peter Wearden, the chemists at Preston Polytechnic who first made them). PW8 was soon replaced by a variant, iso-butyl PW8, which had improved darkening performance, but CG1 continued through to the implementation stage at SOLA.
By luck CG1 and iso-butyl PW8 were also among the easiest compounds to make. In contrast to Heller’s heliochromes, which needed long complicated syntheses and often yielded very small quantities of photochromic dye at the end, Hepworth’s new SINOs could be made in the so-called “one pot process”, whereby the reactants were mixed together in a flask, heated and the final photochromic dye could be easily separated out and purified. This ease of production meant large quantities of Dark Lens SINOs could be made quickly, allowing more extensive work on incorporating the dyes into lenses, and promised a better chance of an economically viable lens.
Although many different Dark Lens SINOs were produced in quick succession at Preston, and certain properties such as the darkening and fading rates of the dyes could be adjusted by changing molecular structures, the colours of these compounds in their activated states were invariably blue or purple. The molecular tailoring needed to make SINOs which coloured to red, orange or yellow was less easy to achieve and less obvious than the techniques to achieve dark blues and control the kinetics.
3.6 Low Catalyst Casting and TEGDM
With a good supply of blue-colouring “Dark Lens” photochromic dyes on stream from Hepworth, work started at Pilkington in 1985 to find viable ways to incorporate these into lenses.
The first candidate for an alternative lens material was based on a crosslinked methacrylate polymer, triethylene glycol dimethacrylate (TEGDM). TEGDM was not an ideal lens material, and it could never be considered a substitute for CR-39 for normal ophthalmic use, but many of its properties were close enough to those of CR-39 to consider for limited application as a photochromic lens.
Positive features of the TEGDM system included the ability to cure the polymer using a low concentration of relatively inactive thermal initiators (which was one key to its compatibility with photochromic dyes) along with the ability to use glass moulds, gaskets, curing ovens, and other ancillary equipment which were already in use for CR-39 throughout SOLA. The lenses also passed the drop ball impact test and had scratch resistance, refractive index and cure shrinkage close to that of CR-39. Crucially for sunlens use, the photochromic TEGDM lenses could be successfully tinted using conventional aqueous dispersed dyes and large-scale sunlens tinting equipment at the SOLMA factory in Italy.
The main negative factor with the new lens was the high reactivity of the monomer and the difficulty in controlling the rate of polymerisation during the cure cycle. TEGDM was a much more chemically active, exothermic material than CR-39 and needed efficient heat transfer and good temperature control during the early parts of the cure cycle to minimise run-away polymerisation and to control exotherm and the rate of shrinkage. For this reason, the curing process during R&D work at Lathom and most of the early scale-up trials at SOLMA used water baths in place of air ovens.
Another consequence of the fast curing, high shrinkage monomer was that it was very difficult to successfully produce lenses with any significant refractive power. On advice from Huan Toh in Australia, the formulation was modified to include a lower shrinkage co-polymer (ATM20), which reduced overall shrinkage from 14% to 12% and helped the reliability of plano casting, but any kind of useful stock lens range still appeared out of reach.
The Low Catalyst Casting technique for incorporating photochromic dyes into lenses was patented in US 4851471 (Maltman & Threlfall)
3.7 SOLA Sensilite Sunlens
Combining the features of the Dark Lens photochromic dyes and Low Catalyst Casting into TEGDM co-polymer, it appeared feasible to make a plano product which would function well as a photochromic sunlens.
Immediately after casting, the unactivated TEGDM lenses containing CG1 and PW8 had a distinct green background colour in the unactivated state, and darkened to a strong blue-purple colour in sunlight. These undesirable colours could be adjusted by overtinting the lenses with a pale brown fixed tint. The result was a sunlens with a 50%T brown tint indoors, darkening to a 15%T blue-grey tint in strong sunlight. The undesirable green background colour was a symptom of the strong UV absorbance of the Dark Lens dyes, which, after correcting with a brown background tint, meant the lens had 100% UV cut-off to 400nm in both its unactivated and activated states – a distinct marketing advantage for a sunlens.
By 1986, the development of proprietary dyes and the TEGDM casting system at Pilkington had progressed to the stage where SOLA’s commercial and marketing personnel could get involved and review lens samples produced in R&D. The result was a decision to market a photochromic sunlens, to be known (in the first instance) as SOLA Sensitive and launch this in 1987. John Bastian, based in Adelaide and recently appointed as Global Marketing Manager was to lead the marketing effort.
The plano sunlens would establish a foothold for SOLA and Pilkington Visioncare in the plastic photochromic business, most likely with a view to following through with an ophthalmic product in due course – either a CR-39 lens with Heller’s Heliochromes (assuming the various technical problems with this approach could be fixed) or some other sort of neutral-colouring lens using mixtures of new compounds from Hepworth and improved lens materials from Project Leapfrog.
The name SOLA Sensitive was quickly revised to become SOLA Sensilite, due to an issue with use of the term Sensitive. The lens would be manufactured at SOLMA in Castiglione Olona, who were involved in the large scale casting and tinting of CR39 sunlenses at that time.
3.8 Casting Development for SOLA Sensilite
Following the decision to implement and launch the photochromic sunlens, SOLA clearly needed to become involved in transferring the small scale R&D casting process from Pilkington to a fully functional manufacturing process for the casting facility.
The first steps were meetings between Pilkington and John McCarthy, Technical Director at SOLA ADC, to discuss detailed requirements for the casting process and make preliminary estimates of manufacturing costs. Following this, in spring 1986 Pilkington personnel travelled to SOLMA in Italy to carry out tinting trials with photochromic lenses cast in the R&D laboratory at Lathom, using the mass-production sunlens dyes and tinting equipment at SOLMA. This phase was to confirm that the particular dyestuffs and tinting conditions used for CR-39 sunlenses were compatible with the photochromic substrate and that acceptable quality could be achieved.
In May 1986, casting trials began in SOLMA, using a water bath purpose-built for the project in Italy and monomer formulations and temperature profiles specified by Lathom. These water bath trials gave worse results than expected from earlier casting runs in R&D, the main problem being a highly variable rate of separations during the cure. Despite intensive effort to control the problem, the water bath cure process could not be brought under control and the decision was made to involve more technical resources and switch the process development task to the Lonsdale Technical Centre.
Work began in Lonsdale in September 1986 and continued into early 1987. Tests were carried out using conventional air ovens instead of a water bath, with the expectation that ovens with properly controlled airflow and new cure profiles based on exotherm measurements, would give more consistent casting performance. Use of existing air ovens would also avoid the large capital cost of designing and building large water baths specifically for the photochromic product. The design of the standard plano gasket was also altered (the so-called feather edge modification) to improve adhesion at the interface between the glass mould, plastic lens and the gasket, where most separations originated.
Good progress was made with converting the TEGDM process to air cure, assisted by access to a new lens processing facility in Lonsdale (the Flexible Open & Shut Line or FOSL), which allowed developmental materials like TEGDM to be handled independently to the normal production lines and had flexibility to be configured to the material’s specific needs.
Although the separation problem was never fully solved and brought under control with air curing, the rate of separations was greatly reduced compared to water cure and those separations which did occur usually failed at the last moment, after the curing oven had been unloaded and just before the moulds were opened. These lenses were not as severely compromised as those which had separated under water in a water bath; the lenses could often be used for subsequent tinting without showing any quality problems and the glass moulds did not need the labour-intensive cleaning required after separation in the water bath.
With larger scale casting now underway, issues started to appear with batch to batch variability of TEGDM monomer. Several drums of monomer with different manufacturer lot numbers were used at Lonsdale, and these were found to show different degrees of success in casting – some batches quite reliable, other batches very poor. Chemical analysis of the composition showed significant batch-to-batch variation in minor components in the TEGDM but there was no convincing correlation between any of these and the observed casting performance. The TEGDM manufacturer was unable to help, pointing out that the monomer had not been intended as a specialty optical material. There were also indications that the batch of TEGDM used for the original water cure trials at Lathom – which had been quite promising. - was by chance one of the best performing lots and not necessarily representative of the standard of TEGDM as a whole.
Another problem identified during the Lonsdale trials was strain in the TEGDM lenses. This was most likely due to inhomogeneous polymerisation and shrinkage during the cure. Some lenses showed mild strain through the centre of the lens, but the most obvious manifestation was a hoop strain which ran around the circumference of the lens. Although visible on inspection of cast lenses the hoop strain would have been almost always edged off the lens during glazing and was unlikely to become an issue for the wearer. However hoop strain was an unresolved problem right to the end of the Sensilite project.
Photochromic casting trials reverted to SOLMA in mid 1987 and continued into early 1988 using the air oven curing process and the modified gasket profile developed in Lonsdale. The results during this second campaign were certainly better than the initial water bath runs, but the casting process was still characterised by day-to-day variability in the rate of process separations and the degree of strain seen in the final lenses. Casting yields in most cases exceeded 90% but there were bad days with lower performance and the causes of these bad days could not usually be identified.
Although thousands of Dark Lens TEGDM planos were successfully manufactured over the course of the implementation trials, the unpredictability and lack of control of the casting process and the variability of the TEGDM raw materials were not considered adequate for an operation intended to mass-produce large numbers of plano sunlenses reliably and at low cost. In February 1988, after seven months of trials at SOLMA, Ian Gillies, the SOLA European Regional Director, halted the photochromic implementation work and suspended the launch of the SOLA Sensilite product.
3.9 SOLA Sensilite Sunlens properties - Dark Lens Photochromics & Low Catalyst Casting
| Lens Material | TEGDM crosslinked methacrylate co-polymer |
| Curing system | Thermal cure ~21 hrs duration |
| Initiator system | Benzoyl peroxide at low concentration |
| Photochromic system | Dark lens SINOs (two compounds) |
| Bleached state colour | Pale green – modified to pale brown by fixed tint |
| Darkened state colour | Dark blue – modified to grey by fixed tint |
| Darkening performance | Rapid darkening. OK for sunlens application |
| Fade-back performance | Reasonable fade rate. OK for sunlens application |
| Temperature sensitivity | Moderate sensitivity. Less activation at higher temperatures, but colour hue stable & acceptable throughout full temperature |
| Fatigue resistance | Good – adequate for lifetime of typical eyewear |
3.10 People involved in the SOLA Sensilite project
- Pilkington R&D
- Bill Maltman
- Mary Ormsby
- Ian Threlfall
- Martin Rickwood
- Preston Polytechnic
- John Hepworth
- Chris Gabbut
- Peter Wearden
- SOLMA (Italy)
- Lino Barbieri
- Mauro Fernandi
- Attilio Antognazza
- SOLA Australia
- Bob Sothman
- Alan O’Leary
- Huan Toh
- John Bastian
- SOLA ADC (Ireland)
- John McCarthy
4. SPECTRUM, SPECTRALITE AND OPHTHALMIC LENSES
4.1 Background to Spectrum technology
During 1987 and 1988, while work was underway at SOLMA to stabilise the Sensilite casting process, Huan Toh and Chong Kok were progressing the development of Spectrum lens materials at Lonsdale. Spectrum technology emerged from Project Leapfrog, with the promise of delivering lens materials which could be tailored to suit either particular product needs (such as higher abrasion resistance or refractive index) or faster, more flexible manufacturing systems.
The Spectrum concept depended on combining different monomers, usually three to six in number, into a blend where each component enhanced some desirable property of the final lens. For example flexibility, tintability, scratch resistance or refractive index of the lens could be controlled and adjusted, within limits, by careful choice of the chemical ingredients and their relative proportions. Whilst making these changes, it was important to retain all the basic performance characteristics needed for a saleable spectacle lens, using CR39 as the benchmark which was well accepted in the market.
The monomers used for Spectrum formulations were from the same general chemical families as the TEGDM and ATM20 materials used for Sensilite. However most of the Spectrum ingredients were sourced from specialist suppliers in Japan. The monomers were designed specifically for optical applications and were manufactured with better consistency and higher purity than had been available for Sensilite.
An attractive feature of Spectrum chemistry was the ability to cure the monomers with UV light as well as by more conventional heat curing in ovens. By the late 1980s, UV curing technology was becoming well established in the electronics, surface coatings and printing industries, but was generally used to produce relatively thin films, typically one-thousandth the thickness of a normal spectacle lens. Developing new technology and processes to successfully manufacture thick lenses by UV curing was definitely not a trivial matter and significant R&D effort had to be devoted to overcoming a wide range of technical challenges. In particular the lens application meant the end product needed to achieve a consistently high level of clarity, whiteness and must be totally free of contamination, strain and optical inhomogeneity.
One of the keys to making viable UV cured spectacle lenses was the choice of initiator. The common classes of photoinitiators used by other UV curing applications were generally found to be unsuitable for making lenses. Using conventional photoinitiators in a Spectrum formulation, the result was usually a lens with uneven cure throughout its bulk, high internal stresses and poor optical quality. There were also other problems such as unacceptable yellowness and poor stability to weathering.
However by choosing a unique and relatively inefficient type of photoinitiator (Vicure 55) it was found that Spectrum formulations could be successfully UV cured to produce both plano and powered lenses without the drawbacks seen with the more conventional classes of initiators.
The UV technique eventually developed for Spectrum lenses was actually a hybrid UV – thermal curing process, which required mould assemblies to be irradiated first with UV, then immediately heated in an oven for a period of time. Heating for 30 to 60 minutes was needed to cure the lenses hard enough to be successfully extracted from the glass moulds, but this was much shorter than the 16 – 21 hour period needed to cure CR-39. The SOLA process differed from other industrial UV curing processes since these generally do not require any heating at all.
4.2 Applications of Spectrum technology
SOLA’s Spectrum technology was launched into the market in 1991 as the mid-index Spectralite lens, but before its eventual commercial release, the Spectrum concept went through a few different manifestations. Between 1987 and 1989 several potential applications progressed a long way through development at Lonsdale, but never came to commercial fruition.
Fast cure cast-to-prescription opportunities had been one of the primary motivations to investigate the UV curing capabilities of Spectrum. This was driven to a large extent by competitor activity and market intelligence in the late 1980s which suggested that small scale, inexpensive in-office casting equipment was about to swamp the market and alter the way the spectacle lens business operated in the North America. This never eventuated, but SOLA took the threat seriously at the time and began investigating fast-cure technologies.
Separately, in the mid to late 1980s, the market for abrasion resistant plastic lenses was increasing rapidly. To meet this demand, SOLA had chosen spin coating as the most economic way of mass-producing hardcoated CR-39 products and launched its PermaGard brand. Petaluma developed semi-automated coating equipment which could be configured for either semifinished or finished products and a customized liquid coating resin which suited CR39 and the particular needs of the spin coating process.
Despite many successful advances with PermaGard, there were ongoing challenges in maintaining a consistent supply of lenses to the market and in servicing demands for an increasing variety of coated products. Some of the problems related to SOLA’s relative inexperience with the new spin coating equipment and the coating resins in use at the time. This could result in frequent machine breakdowns, periods of low production yields, or product quality issues such as crazing or delamination of the coatings. Those sites involved in manufacturing PermaGard products needed to devote a large amount of technical and quality control resources to supporting their coating operations.
In addition, each spin coating machine had a high capital cost and was relatively complex for an organisation the size of SOLA at the time. For a time it was difficult for the engineering function in Petaluma to roll out enough new coating machines to meet demand at the established coating sites in USA and Australia, irrespective of the need to implement spin coating at other sites such as Wexford or Brazil. It also would be difficult to provide the necessary level of technical expertise to support other SOLA sites, given the known sensitivity of the spin coating process at the time.
One solution to the various issues with PermaGard was to exploit the abrasion resistant properties of Spectrum monomers and cast lenses which did not need coating at all. With a suitable choice of monomers, it was possible to formulate Spectrum lenses which were similar to CR-39 in most of their properties, but had abrasion resistance in the uncoated state equivalent to that of CR-39 with PermaGard. These became known as the Inherently Scratch Resistant (ISR) formulations.
Although it was possible to match the performance of PermaGard/CR-39 (as measured by the Taber Abrader, a standardised laboratory abrasion resistance test), it was found that the Spectrum formulations with the best abrasion resistance were not suitable for casting powered lenses, or indeed any thicknesses greater than about 2mm. Since the main target was ophthalmic products, this was a show stopper. A de-tuned, modified monomer formulation was eventually chosen, known as Spectrum B. Spectrum B had material properties generally similar to CR39 and provided an acceptable compromise between enhanced abrasion resistance (still close to PermaGard in laboratory tests) and the capability to be successfully UV cured as finished lenses across a +6 to -6 D power range.
Despite their limitation with powered lenses, the true Spectrum ISR formulations could be used to make good quality planos with a uniform cross section and thicknesses around 1.8 – 2.0 mm. This capability was used to validate production-scale UV curing equipment, and also to progress the development of photochromic lenses beyond the stage reached with Sensilite at SOLMA.
4.3 The Simpson Module
Alongside efforts to refine the Spectrum monomer and photoinitiator systems, another significant work package at Lonsdale aimed to develop the equipment needed to manufacture lenses by UV curing. As with the photoinitiators, the UV curing industry of the time was focused on curing only flat products with a very different disposition to thick spectacle lenses, so no ready-made equipment was available off-the-shelf and industry suppliers were unable to offer much practical advice.
Several generations of prototype hardware were built in quick succession by the R&D engineers at Lonsdale. These prototypes were used to prove the UV curing concept, to understand the operation of UV lamps in the unusual face-to-face orientation (thought to be needed for simultaneous irradiation through both front and back molds) and to test associated new technologies needed for UV curing, including PVC gaskets, side filling techniques and the requirements for polished glass moulds.
The final stage of this development was the construction of a purpose-built automated machine which would take care of filling and UV/heat curing the mould assemblies, then would strip the PVC gaskets and feed the mold/lens assembly into an existing cleaning apparatus (the FOSL, or Flexible Open & Shut Line). This equipment was installed in Lonsdale R&D, and was built by Simpson Automation, an industrial automation firm in Adelaide, hence its enduring name – the Simpson Module.
The intention at the time was to use the Simpson Module as a prototype to de-bug, refine and specify a highly automated UV curing process, then to build turn-key production modules which could be supplied to SOLA manufacturing sites. The high level of automation in the Simpson Module concept meant the machine needed only one operator to load empty assemblies into one end of the machine, with fully cured, cleaned mold/lens assemblies being delivered into an Open & Shut clean room at the other end without any manual handling in between.
4.4 Spectrum UV380 ISR sunlenses
At the time of its development, the Simpson Module was intended for production of inherently scratch resistant stock lenses which did not require hardcoating. With so much novel manufacturing technology combined into one process – not only Spectrum monomers & UV curing, but also new monomer preparation equipment, PVC gaskets, three-port side filling and the new FOSL cleaning line, it was necessary to run a substantial pilot production exercise to prove these processes before making any commitment to manufacturing or commercial operations. The pilot phase would be expensive if stock lenses were produced – since there was little opportunity to market the small number of Spectrum B stock lenses which would be generated.
However a solution came via Brian Staples in the Sales & Marketing function in SOLA Optical Australia, who was able to negotiate with a sunlens customer in Sydney to purchase a 150,000 pair batch of tinted planos in Spectrum ISR material with a 380nm UV cut-off (hence UV380 ISR). These were offered under normal commercial arrangements as CR-39 look-a-like lenses, which passed all relevant Australian Standards but with no claims of improved abrasion resistance. This would recoup some of the costs of the pilot production and the fall-back position if Spectrum technology or Simpson Module failed would be for SOA to fulfil this order in conventional CR-39.
In 1989 a small team of R&D and SOLA Optical Australia production staff undertook a pilot production campaign using the Simpson Module to produce a total of 380,000 pieces of Spectrum ISR sunlens planos which were tinted, inspected and packaged in SOLA Optical Australia, shipped to the customer and fitted to brand-name sunglasses for the upcoming summer season.
The UV380 ISR exercise proved the viability of most aspects of the Spectrum UV curing technology for day-to-day production, achieving yields of 90% clear lenses and 95% in the sunlens tinting process Some deficiencies were identified with the operation of the Simpson Module, and these experiences facilitated improvements in areas such as reliability of sensors within the Simpson Module, positioning of assemblies on the metal transportation bars, automatic side filling techniques and gasket stripping.
People involved in the UV380 ISR & Simpson Module project:- Michael Gilbert
- Colin Lewis
- Randal Engel
- Ian Threlfall
- Julie Barrett
- Jackie Morgan
4.5 Spectrum ISR Photochromic sunlenses
Concurrently with the validation of the Simpson Module for ISR sunlenses and the development of a formulation for casting Spectrum B stock lenses, work was underway in R&D during 1988 and 1989 to incorporate the Pilkington “Dark Lens” photochromics into Spectrum ISR monomers. This was expected to produce a photochromic sunlens generally similar to the proposed Sensilite lens, but with improved Spectrum material properties and avoiding the unreliable thermal cure.
Whilst attempting to make photochromic Spectrum lenses, analogies were encountered which mirrored earlier experiences with photochromic CR-39. The Vicure 55 photoinitiator, which had been the key to successfully UV-curing clear Spectrum lenses, was found to be highly antagonistic to the SINO photochromic dyes and rendered the cured lenses non-photochromic in the same way that IPP or SIP had destroyed the dyes in CR-39.
Eventually alternative types of photoinitiator were found which were capable of properly curing the photochromic monomer without destroying the photochromic effect. Some of these initiators had been tested in the early phase of Spectrum and found to be unsuitable for clear lenses, but on re-evaluation were found to be good candidates for photochromic formulations, where the UV absorption characteristics and cure kinetics were significantly different.
Another material was Lucirin TPO, a new class of photoinititor which appeared on the market for the first time while Spectrum ISR photochromics were under development. Lucirin TPO was found to be the preferred initiator and was used with all subsequent Pilkington photochromic formulations based on Spectrum or Spectralite.
One aspect of the development project for photochromic Spectrum ISR was to analyse the likely manufacturing cost of the lenses. Once the formulation and technical framework were defined on a laboratory scale, a financial modelling exercise showed that the higher price of the Spectrum monomers, combined with polished glass molds, single-use PVC gasket and amortization of the UV hardware made the Spectrum ISR photochromic very much more expensive than its Sensilite predecessor, and clearly uneconomic within the cost structure of the sunlens business. Interestingly, the cost of the photochromic dyes, which had consumed so much R&D time and money, was quite modest on a per lens basis and did not influence the financial viability of the product.
With such high product costs projected, development of sunlenses with Pilkington PW8 & CG1 dyes was halted and focus turned to longer term ophthalmic opportunities.
4.6 Spectrum ISR Sunlens properties
| Lens Material | Urethane methacrylate / acrylate co-polymers |
| Curing system | UV cure, then 45 minute duration heating |
| Initiator system | Lucirin TPO photoinitator at low concentration |
| Photochromic system | Dark lens SINOs (two compounds) |
| Bleached state colour | Pale green – modified to brown by fixed tint |
| Darkened state colour | Dark blue – modified to grey by fixed tint |
| Darkening performance | Rapid darkening. OK for sunlens application |
| Fade-back performance | Reasonable fade rate. OK for sunlens application | Temperature sensitivity | Moderate sensitivity. Less activation at higher temperatures, but colour hue stable & acceptable throughout temperature range |
| Fatigue resistance | Good – adequate for lifetime of typical sunlens |
4.7 Introduction of Spectralite
In the late 1980s and into the 1990s, following technical developments made at companies such as Gentex, polycarbonate lenses began to take an increasing share of the plastic lens market in North America. Polycarbonate lenses had several marketable benefits over CR-39, principally high impact resistance and low density (specific gravity) which made it possible to promote lenses as safer and “thinner and lighter” than CR-39 for a given prescription. There were also some downsides – lower optical quality, difficulties in tinting and problems processing polycarbonate lenses with conventional Rx laboratory equipment, but nonetheless polycarbonate’s market share was increasing.
SOLA did not have a polycarbonate manufacturing capability at the time, and used two strategies to counteract the threat to CR-39’s dominance in North America.
One approach was development of the ATOM polycarbonate moulding process at Petaluma. The ATOM injection-compression technique was another output of Project Leapfrog and produced semifinished lenses with superior optical properties to injection molded polycarbonate from competitors.
SOLA’s second approach was to use Spectrum technology to develop a UV cured mid-index lens material, branded Spectralite. The refractive index of Spectralite, at 1.54 was not as high as polycarbonate’s 1.59, but it had low density, better optical and chromatic aberration properties than polycarbonate and Spectralite lenses could be easily tinted and processed using conventional Rx laboratory equipment “just like CR-39”. Using flattened, aspheric lens designs for parts of the prescription range, Spectralite could have the “thinner and lighter” benefits of polycarbonate competitors without the unwanted drawbacks of the poly material.
Furthermore the UV curing process used to manufacture Spectralite allowed glass molds to be re-used several times per shift, rather than once every 24 hours as was the case with CR-39. This meant a lower inventory of expensive slumped molds was needed to manufacture progressives, or alternatively new products could be cast in larger numbers and introduced more quickly.
Spectralite products were first introduced in 1991. Semifinished products were manufactured at SOUSA as VIP Gold and XL Gold progressive designs, along with an aspheric single vision semifinished. SOA in Lonsdale manufactured the aspheric Finished Single Vision range, initially using the Simpson Module.
4.8 Spectralite Photochromics
Following the demise of Sensilite and Spectrum ISR photochromic sunlenses, Pilkington R&D and Professor Hepworth’s team at the University of Central Lancashire re-focussed their efforts onto photochromic dyes which would give neutral-colouring lenses suitable for ophthalmic applications.
During the period 1989 to 1991 while SOLA was developing and implementing Spectralite, Pilkington & Hepworth gained significant experience in identifying the chemical structures needed for neutral-colouring systems of dyes and the techniques needed to synthesise the required compounds.
As with Heller’s Heliochromes, Hepworth’s photochromic dyes coloured to distinct colours – blue, green, orange or yellow, and mixtures of several colours were needed in specific proportions to achieve true grey or brown colouring lenses. Hepworth’s system used SINO photochromic dyes to provide the purple, blue or green components, and Chromene dyes to cover the orange and yellow portion of the spectrum.
As Pilkington and Hepworth progressed their new dye developments, small quantities (usually much less than 1 gram) of the more promising materials were sent to Lonsdale R&D for small-scale test casting in Spectralite lenses, using the photoinitiator and cure processes identified for Spectrum ISR.
Extra impetus for ophthalmic photochromic development was provided by the release of Transitions Plus in 1992. The first generation Transitions lens had been launched in 1991, which changed from a pale brown to a moderate greyish-blue colour in the sun. Transitions I had limited appeal, but it was followed fairly rapidly in 1992 by the second generation product, Transitions Plus. Transitions Plus used a multiple dye system to achieve better neutral colours.
By this time SOLA R&D and Pilkington had developed a photochromic lens formulation based on Spectralite and three of Hepworth’s dyes, which was very competitive with Transitions Plus. In side-by-side comparisons in natural sunlight the SOLA lens went somewhat darker than Transitions Plus, and maintained a more neutral colour during fade-back (Transitions Plus tended to fade through yellow hues).
The SOLA lens also had better fatigue performance, due to the photochromics being distributed through the bulk of the Spectralite polymer rather than incorporated by surface imbibition.
4.9 Front Surface casting with Spectralite
One drawback of the Pilkington/Spectralite lens was related to the cast-in incorporation method. Photochromic dyes were distributed through the bulk of the lens, resulting in higher prescriptions having a “bull’s eye” effect when the lens was activated and thicker regions of the lens would appear noticeably darker than the thinner parts. This applied to both plus and minus powers, but was absent on Transitions product whatever the prescription.
A laboratory scale solution to the bulls eyes was developed which was applicable to semifinished blanks, including progressives. This was based on the UV curing capabilities of Spectralite and was known as Front Surface Casting.
The steps were as follows:-
- A semifinished blank was cast, using normal clear Spectralite monomer, and a reduced (partial) UV and heat cure regime. The blank was cured sufficiently to be opened and handled without damage.
- The clear Spectralite blank was re-assembled into a PVC gasket, using the lens as a “back mold”, the same glass mold as previously as the front mold and leaving a cavity of around 1.5 to 2mm thickness was between the two.
- The cavity was filled with photochromic Spectralite monomer and the assembly was UV cured a second time, irradiating only through the front glass mold which fully cured the Spectralite.
After removing the gasket and glass front mold, the photochromic layer adhered strongly to the clear Spectralite blank and being of uniform thickness did not show any bulls eye effect after activation, whatever prescription the blank was subsequently surfaced to. The clear-to-photochromic interface was found to be a very durable and survived all normal weathering tests, surfacing and coating processes without failure.
Using a progressive glass front mold, it was possible, on a laboratory scale, to make photochromic Spectralite progressives, up to 8-base with 300 D addition, which had superior photochromic response and better fatigue resistance than the Transitions Plus benchmark of the time. However, major process development and significant product testing would obviously be needed to commercialise a photochromic progressive, or any ophthalmic product based on front surface casting.
4.10 Spectralite Photochromic lens properties (Pilkington system)
| Lens Material | Spectralite urethane/methacrylate co-polymer |
| Curing system | UV cure, then 45 minute duration heating |
| Initiator system | Lucirin TPO photoinitator at low concentration |
| Photochromic system |
Three “Hepworth” dyes:- AN11 (green colouring SINO) 12B-01 (orange colouring chromene) 27B-37 (yellow colouring chromene) |
| Bleached state colour | Pale brown fixed tint ~87%T |
| Darkened state colour |
Dark grey – bulls-eye in high prescription with direct casting. – no bulls eye if Front Surface casting used. |
| Darkening performance | Rapid darkening. Comparable to Transitions Plus |
| Fade-back performance | Good fade rate. Better than Transitions Plus |
| Temperature sensitivity |
Moderate sensitivity. Less activation at higher temperatures, but colour hue stable & acceptable throughout temperature range |
| Fatigue resistance | Good – appears adequate for lifetime of typical prescription (although no wearer trial was performed) |
4.11 Wrap-up of Pilkington Photochromics
The R&D development of the three Hepworth dyes and front surface casting into Spectralite occurred at the time when Pilkington was preparing their vision-care business for sale, and Transitions (backed by PPG and Essilor) were gaining traction in the market with Transitions Plus and their Comfort Lens concept.
If SOLA were to manufacture and market photochromic lenses using its own proprietary technology, the company would need to be in for the long haul. It would be necessary to:
- Prove the viability of photochromic Spectralite casting in manufacturing, including development of front surface casting if the product required this.
- Develop a new capability, or a relationship with an external body (e.g. a university) for an ongoing photochromic dye development programme.
- Develop a supply channel with commercial suppliers of photochromic dyes.
- Support long-term, ongoing R&D expenditure to keep pace with future developments from other players such as PPG, Transitions and Tokuyama.
In the climate of the time, this was a risky and unpalatable commitment and decisions were made to sell or licence the portfolio of photochromic technology which was applicable to spectacle lenses to other players in the field, and for SOLA to enter commercial agreements as a lens caster with Transitions to supply SOLA Transitions products. Given the continuous development of photochromic systems at Transitions (now up to Generation 6) and the strength of the Transitions brand globally, this seems to have been a wise strategic decision.
SNATCHING DEFEAT FROM THE JAWS OF DEFEAT: THE LAMINATE ADVENTURE
By Peter Coldrey
1989-1992 Director of Research SIHL
1992-1997 V.P. Research, Development and Engineering, SOUSA
1997-2000 Director of Technology, SOLA Sunlens Division

Excerpt
These recommendations in normal SOLA times would have been implemented in a flash, in fact, if it had been normal times the extra base curves in Percepta would have already been in the market. Here was a project that would deliver by 2003, at minimal risk, a US$10 million boost to our high margin product sales. But unfortunately in late 1999 times in SOLA were not normal. Our systems were broken, key people were missing or emasculated and we were at a time when a three month time horizon was long term. And it was just not possible to engage the SOLA organisation at the time in such a worthy endeavour.
From around 1991 Tom Balch in SOLA USA started to champion the idea of making finished multifocal lenses via lamination. The concept involved a back wafer which would carry single vision and cylinder power and the front wafer which contained the multifocal correction and for some elements would additionally contain some single vision power.
Prior to sticking the wafers together the back wafer could be rotated relative to the front to align the cylinder power to that required by the script. Since cylinder power is prescribed to 5 degrees the rotation facility meant that with the two wafers, 72 different scripts could be made. The ability to mix and match front and back wafers provided additional opportunities to make multiple scripts from a smaller number of pieces. The net result was that the laminates system eventually developed by SOLA contained less than 250 wafers and could be used to fill many thousands of scripts in the range from -4.00 D to +4.00 dioptres to a maximum of 2.00 D cyl and a progressive reading element up to 3.00 D. (this represents around 80% of all prescriptions)
The great market opportunity identified was to use this system to give Lenscrafters in the USA the opportunity to provide anti-reflective (AR) coated lenses “in about an hour” in more than 700 stores. This would be possible by AR coating the front of the front wafer and the back of the back wafer in large factory based AR coaters. The concept involved an inventory of AR coated wafers in each Lenscrafters store and for one of their on-site staff to make the laminate on-site to meet the prescription of the person who just walked into the store. The lamination process clearly needed to be very simple as it would have to survive in the face of a diversity of people of widely varying capability and limited experience.
By the time Peter Coldrey arrived in the USA in August 1992 to take over from Tom Balch, who moved to Younger, the laminates project was the biggest in the USA R&D portfolio. During Coldrey’s 5 year stay from 1992 to 1997 the R&D expenditure grew to more than US $4 million p.a.
It was a fantastic R&D team full of confidence from many previous new product introductions which had provided the foundation for SOLA’s rapid growth. In 1992 Spectralite was really starting to make an impact in the USA market and in 1994 SOLA USA launched Spectralite in Transitions, the first “high index” Transitions product. This was a very high margin product and sales in the first year went close to $20 million, representing nearly 10% of SOLA Optical USA’s total sales at the time.
There was Dave Spector an engineer with limited formal training but a genius in his own way. He had an unparalleled knowledge of how everything worked and if he needed some special bit of equipment he could usually build it. There was Rock Weir, a locally trained engineer, who could design and have built the most ingenious of devices --- nothing was beyond his scope. Luan Modglin looked after all mold specification and she was supported by a great team which included Carol Parker, Mike Shannon and Herbert Graff. Of course, the SOLA USA legends Eric Barkan and David Sklar provided all lens design for the system. Then there was Paul Coates who was a graduate in chemical engineering from Cambridge University (U.K.) with a PhD from MIT. A great and most capable guy who worked on the nuts and bolts of lamination. He was very ably supported by Linda Davidowski, who always seemed able to produce a first quality lens by lamination. Another key member of the team and the Project Manager in its later stage was David Ambler. He was an ex SOLA Australia employee who emigrated from Australia to the USA. David was a lens casting expert and a good project leader -- he had been a key driver in SOLA’s earlier successes with Spectralite and Spectralite in Transitions. For the AR coating we had Steve Machol, who had a dedicated $1 million Balzer’s vacuum coating system at his disposal and Clive Burton, another ex SOLA Australia employee worked to develop new sputter coating AR technology. And on the polycarbonate side was Jeff Kingsbury and Rob Juul. Fantastic guys who always seemed to find a way to make things better (and fun). Tragically Jeff died from a brain aneurism in 1999
The project was large because there were numerous technical challenges to overcome. Not least of which was having to cast a finished lens, incorporating a progressive element at a thickness much thinner than had ever been achieved before. A complete set of new molds had to be developed and there was an additional requirement that the interface curve of all wafers had to match precisely.
Bulk AR coating was not a well established process at the time, particularly for very thin lens elements and considerable development was needed on this front.
Then there was the matter of sticking the two laminate elements together to make a lens. UV curing glues were found to be quite effective, although special technology was developed to provide adequate adhesion. Since the lens needed to be UV protecting, it was necessary to include the UV absorber only in the front wafers and cure the laminate through the back wafer to which no UV absorber was added.
The laminating module was a key element of the system and this went through many iterations before Coburn Optical were charged with the job of producing a manufacturable system. They produced an excellent unit for under $10,000 per module. The design enabled the operator to laminate two lenses at the same time in about 5 to 6 minutes.
There were numerous other challenges met and overcome by the R&D team. One was a peelable interface film to ensure a clean interface surface was presented at the point of lamination. It was judged that the Lenscrafters staff would have insufficient skill to be able to appropriately clean an unprotected interface surface.
Another more difficult problem was that the laminates changed their base curve with humidity changes - this didn’t affect the overall power of the element, but it meant that the interface curved moved from the design value and the elements could not be successfully laminated. This issue was solved by individually packaging each wafer in a very high moisture barrier pouch. It was an elegant solution but added to the cost and the barrier material was quite stiff so that the packaged wafers did not pack all that neatly together.
Matrix was a major R&D and market development undertaking but by around 1996 we had a system which worked and individual store trials commenced in selected Lenscrafters locations. We were also able to invite customers to demonstrations where we would make up their script in 10 minutes or so and they would leave inside 30 minutes with a new pair of multi-focal, AR coated spectacles.
It wasn’t all good news as in the rather large time that it had taken to develop a workable system (in CR39), Lenscrafters had moved its market away from cast CR-39 lenses towards polycarbonate. This was not all that surprising as they made more money from dispensing a polycarbonate lens and the quality of polycarbonate lenses had increased rapidly in recent years (on the back of cleaner polycarbonate being developed for the CD industry). The other major thing going for polycarbonate is that it has outstanding impact performance and litigation for lens fractures is a major issue for USA spectacle lens dispensers. Lenscrafters went as far as not allowing its staff to prescribe anything but polycarbonate lenses for customers who were 18years old or younger.
So while the CR-39 laminates system was being brought to market, we had a parallel program trying to invent a process to mold polycarbonate wafers with a centre thickness of 1mm. We needed a centre thickness this small so that the finished lens would have a centre thickness around 2 mm which was around the centre thickness of conventionally made polycarbonate lenses. There was a guy, Nick McCaffery, who used to work for Moldflow and later SOLA R&D in Australia, who had an injection moulding simulation package which he would run to “prove” that it was not possible to mold a wafer thinner than 1.5mm in the centre without getting physical distortions. Jeff Kingsbury then would mold a wafer at around 1.4mm without distortions and Nick would go back to the drawing boards and define a new limit. To cut a long story short, despite Nick’s many claims that it was impossible, Jeff developed a process where he could mold polycarbonate wafers sufficiently thin.
Well the rest is history --- the laminate system was rolled out in more than 700 Lenscrafters stores in early 1999 with an investment in laminating modules alone of around US$7 million. The sales target was around 1.5 scripts per day per store. The value of the lens elements for SOLA for each script was about US$50 so that if sales of 1.5 scripts per day per store was achieved the aggregate daily sales would have been around US$50,000 and yearly in excess of $15 million -- not a bad earner.
In reality the required sales penetration was not achieved and while the system limped along for many months, Lenscrafters eventually abandoned it.!!
So that is the first defeat but unfortunately not the last.
At about this time (1995/6) we were bringing the CR-39 laminate system to fruition, SOLA also saw a major opportunity for polarised prescription lenses and started work to produce their own. The polarising effect is achieved in a lens by incorporating a thin polarise foil in the middle of the lens. This can be achieved in two basic processes: “cast in” or lamination. (There are other options for polycarbonate which will not be discussed here.)
Cast In: This process is suitable for thermosetting materials like CR-39 and Spectralite. A curved polarised film is held in the lens gasket assembly in a way which keeps it close to the front mold surface. The mold assembly is then filled and cured in the usual way. Notches are made in the polarised film to allow free flow of the monomer around the back and front of the wafer during filling. This is a tricky process but successfully implemented by a number of producers
Lamination: This process consists of having a front and back wafer of the required lens material and gluing the polarised film between the wafers to make a lens. The technique can be used for all materials but is most commonly used for polarised glass lenses. By using powered wafers it is possible to make prescription lenses as well as planos. Until SOLA started developing its ophthalmic lens lamination system the lamination method for making polarised lenses was confined exclusively to plano lenses (sun lenses) and then mainly used to make glass lenses.
More than 95% of the difficulty involved in the lamination method lies in making the wafers correctly. The rest of the task is almost trivial. And whilst it was not the target of SOLA’s lamination project, in bringing a lamination system to fruition it had, as a side benefit, provided a simple route to manufacture all its lens designs with a polarising option. From 1995 we were able to make polarising lenses via lamination in the laboratory but gaining reliable supply of the polarising foils in the base curves needed for our system proved difficult. There were very limited supply options in Japan and, at the time, most chose not to deal with SOLA. The few foils we did manage to procure were used to develop a process and make polarised progressive lenses for trail by SOLA R&D staff. I still have a pair of polarised progressive spectacles made from this period that they served me well until my reading correction grew beyond what was provided by this prescription
By around 1996 we had established an improving supply position for the polarising foils and a decision was made to make a polarised version in our lead progressive lens at the time which was “Percepta”. This product would be supplied as a semi finished blank which simplified the manufacture somewhat compared to a standard Matrix product. With a semi finished product the “back wafer” had a centre thickness of a few mm rather than the 1.2 to 1.5 mm typical of most Matrix wafers.
Katherine Ingram was made project manager for this project. She was a very talented young NZ scientist who was living in the Bay area with her husband. She did a great job bringing Polarised Percepta to market but as is often the case some serious mistakes were made in launching the product.
Jim Cox, the President of SOUSA at the time, made a unilateral pronouncement at an annual Sales Conference that Polarised Percepta would be available within some ridiculously short time period. This may not have been so bad if the Sales people had not believed him. Despite protests from the development people that the promised timing was unachievable, Jim stuck with his date presumably to ensure that the project would receive maximum priority in R&D. In the event the product was launched around the start of 1998, about 3 months after Jim’s promised date and this was seen as a great failure because supply had been promised 3 months earlier to all our customers. Our sales force was embarrassed by the late delivery and the R&D lab was seen to have failed. The product itself, after some early teething problems, actually performed well in the market (see later), but in the wake of her major “failure” as a Project Manager, Katherine resigned from the company to its great detriment.
In mid 1999, I was commissioned to explore how SOLA could best participate in the rapidly growing polarised lens segment of the market. Matrix was struggling and I identified an excellent opportunity for SOLA to leverage its extensive lamination technology to make a wide range of polarised lenses. Thus, even if Matrix itself did not succeed SOLA would still have an alternative product avenue through which it could get a solid return on its massive laminates R&D investment.
I found to my amazement that SOLA had captured approximately 5% of the North American polarised ophthalmic lens market with its polarised Percepta product. This was achieved with very limited marketing support and no promotion. The polarised offering was only 3 base curves when regular Percepta has 6 and average year-to-date margins for the polarised product were running at running at 52%.
My review included many recommendations but the principal one was to build on the success of polarised Percepta. To add extra base curves to the Percepta range and to offer other premium SOLA designs with a polarised option. At the time these designs were ASL, an aspheric single vision product and “Spazio”, a high base curve product designed as an ophthalmic sunlens product. I also recommended that the polarised be extended into other materials including Spectralite High Index and Polycarbonate.
There was no impediment to offering polarised products in Spectralite as the lamination system had been proven in this material since 1995. By 1999, lamination in polycarbonate was also established.
These recommendations in normal SOLA times would have been implemented in a flash, in fact, if it had been normal times the extra base curves in Percepta would have already been in the market. Here was a project that would deliver by 2003, at minimal risk a $10million boost to our high margin product sales. But unfortunately in late 1999 times in SOLA were not normal. Our systems were broken, key people were missing or emasculated and we were at a time when a three month time horizon was long term. And it was just not possible to engage the SOLA organisation at the time in such a worthy endeavour. Barry Packham, Matthew Cuthbertson and I as a group all tried valiantly to make it happen and we failed.
I vividly recall my words at a presentation I gave seeking support for the proposal –
If SOLA is unable to capture an opportunity of this quality then the company as we know it to-day (high margin/high R&D spend) will not survive !!!!!
And alas in failing to realise the fantastic potential SOLA’s lamination technology offered to developing an across-the-range offering of polarised products we looked again at defeat, when victory was so near!!
GOLDFISH/ENIGMA– fantastic idea but abject failure Steve Daly

Goldfish/Enigma was a revolutionary product developed by SOLA that offered a lens that was different.
The SOLA marketing literature for Enigma stated:-
- Surround vision for the real world
- Flat eyewear results in ordinary vision. Curved eyewear results in extraordinary surround vision (40% wider field of view, less chromatic aberration in polycarbonate, etc). Introducing Enigma, the first single vision eyewear to follow the natural curvature of your eye.
- Curved, convex, contoured in shape. Enigma is a design revolution combining ‘Contour Optics by SOLA’, a patented new lens technology, and exclusive custom-designed frames by SAFILO. Enigma provides the most precise, unrestricted vision available in single vision eyewear. The result is a look that will change the shape of eyewear forever.
Goldfish/Enigma met the criteria of how to differentiate visually and performance wise SOLA’s product from competitor’s. Mike Morris started the classic Goldfish concept with spherical lenses placing the center of rotation of the eye at the geometric center of the lens, so avoiding off-axis error. Colin Perrott found the breakthrough.
In Colin’s words:
I stumbled on the wrap-around prescription lenses concept that's gave SOLA a real way to look and be different as a company. I got this idea ....going home for lunch, sitting, saying, 'How can we do something different?' We knew we needed to be able to come up with a product that in four seconds you could explain to somebody what it was, and they'd want to have it. But what the product would be was not at all obvious. So, this is how it came about…..
The feature of early summer in Menlo Park area is you get nice days, and there are lots of people out riding bicycles and the like. So I noticed one day that all these people wear a lot of stuff they don't need to have. You know, just because they want to look cool. And it seemed to me that we could make, without any problem - intellectually without any problem - products which would wrap-around, just like the Oakley type sun-glasses, or make shades, whatever. And it probably took twelve months or so of fiddling around with the mathematics to work out how to produce reasonable optics in such a product. Meanwhile, nothing was being done in either the R&D facilities. It was just me playing around in Menlo Park”.
All of Colin’s work was about surfaces that were not rotationally symmetric and flatter in the middle to avoid the bug-eye effects. In final form, these were not even close to conics. They were spiric designs and gave astounding optics. Steve Spratt was the detail man who converted concept and general calculations into precise surfaces.
The marketing program was led by Peter Joy, the talented marketing executive with limited optical knowledge but great enthusiasm for the project. He focussed on distinguishing the visually different lens. Paraic Begley led the technology development.
After initially working with a Japanese frame manufacture, a joint venture was formed with Safilo where SOLA provided the lenses, Safilo provided the frames and the lenses processed through SOLA USA Rx labs.
Technology wise, major innovation was required to deliver the product.
The lenses – the design called for a non-rotationally symmetric surface – it was toric and so it was a new surface invented and designed by Steve Sprat in SOLA USA. Apart from the innovation of the actual surfaces, to realize the optics was a new invention.
Once the optics were defined, the results from the initial casting trials and with the target market of the USA, led to a polycarbonate material specification. Polycarbonate has to be molded and because it was highly curved, development was required on the molding equipment, molding tooling, inserts and the molding process. In all these areas there was innovation. Initially Arburg injection molders were used to try to produce the lenses but ultimately the manufacture was done with Ximtar (ie with technology significantly different from standard injection molding which was developed by SOLA USA for producing polycarbonate Matrix wafers).
Because of the high curve and the flow of the material etc, all the tooling design was unique and had to be developed in-house. The insert development was another area of innovation as the parts were non-rotationally symmetric. So there was a lot of work required first of all in milling the metal molds because the milling machines were not fundamentally capable without the right milling strategy to deliver the required surfaces. During development of the internal capability in SOLA USA, an external consultant in the USA who had done work for NASA, was used.
It was a great challenge just to machine the parts and then to polish them. Michael Gall and his team cut their teeth learning how to polish non-rotationally symmetric surfaces. Understanding the metrology and capability of the non-rotationally symmetric parts and how to measure them to ensure they were within tolerance required invention. Historically SOLA only measure parts in the centre and distance but for Goldfish, SOLA had to make sure the whole surface was compliant. Polaroid Corporation helped develop all the algorisms which were the precursor to the software now used in CZV for processing Freeform. A lot of these algorisms which are now BDP started in Goldfish.
Hard coating highly curved parts was also a real challenge with the development lead by Dave Diggins. Brandon Yip worked on getting an even color and properties for the AR coating. Fung Chen developed a method of applying edge color to the lenses. Ian Bateman and then Steve Nedomansky were responsible for manufacturing.
Dave Carr, in SOLA USA, did all the development of the special edging equipment required to edge the highly curved parts as the normal edgers for flatter parts did not work; he worked with Safilo to define the frame specifications as new frames had to be developed to hold the lenses.
The issues with Enigma were:-
- They look cosmetically OK for low powers but not for high powers where the cosmetic appearance made one look like one had goggles on. The general customer feedback was that they looked cool in low powers or Sun Rx but not for high powers - this was a real stumbling block.
- The pricing in the market - they were targeted at single vision wearers, not progressives where you have higher added value, so the look has to support the price as they were going to be expensive with special frames and special lenses. That is where the whole thing fell down – the product just did not support the ambition of a superb looking, trendy, visibly different, high priced offering.
After the departure of Peter Joy, Joan Hollywood who was already working in SOLA USA put together a completely new marketing package to position the strategy a bit more sensibly and to get more frame partners. Joan was not up to the task.
The project started early 1996 and was abandoned in Dec 2001. The cost was in excess of US$8 million and there were no $cash returns – however significant spin-off benefits did eventuate - refer last two paragraphs.
The failure was due to both marketing and R&D:-
- Marketing failure. Regardless of the ground-breaking nature of the technology of the product, the key to its commercial success was always going to be in its effective marketing in the US market place. After Peter Joy’s departed in mid-2000, the crucial marketing effort failed to gain any traction.
- The frames were very old fashioned by European standards (according to Jeremy Bishop and that's where SOLA had the labs to process and thus sell the product) and hence, virtually unsalable
- The Rx range was too limited.
Additionally, in the beginning the core marketing and development team defined what Enigma would be - it was going to be a polycarbonate lens with a certain limited range. However it was only at the end of the product development, really only after the first iteration, that it became clear the product look did not support the ambition of the marketing objectives. It was not that SOLA failed to meet a specification - it was that when delivered against the specification it was clear the product, which had not been tested widely enough, was not going to support the marketing ambition – that is a lesson in product development in itself, i.e. getting enough product and testing it in the market before committing to a technology path.
However Goldfish was where SOLA built up all its expertise on manufacturing complicated surfaces. Two very tangible inventions flowed from Goldfish to Freeform:-
- The whole understanding of the metrology of non-rotationally symmetric surfaces
- The ability to make non-rotationally symmetric parts
These precursors shortened the lead-time for developing Freeform capability. Freeform immediately followed on from Goldfish. Freeform became a significant earner for CZV.
CONTACT LENSES Tony Phillips
Pilkington had acquired SOLA in the late 1970s as they could see the end of glass spectacle lenses. The next logical step beyond plastic spectacle lenses was to expand into the contact lens arena.
The problem for a new company entering this area was that it was already hugely dominated by two companies - Bausch and Lomb and Cooper Vision. The only way to enter the market was with a novel product. Fortunately SOLA possessed the technical expertise to do this - the ability to mold lenses at a very low unit cost. This led to the concept of a disposable system. It was further felt that the other factor of appeal would be to aim for an 'extended wear' lens i.e. lenses that could be worn continuously day and night for a month. Thus the aim to make an emphatic entry as a new player into the market was the production of an affordable, extended wear lens that could be worn for a month and then replaced with a new, sterile lens.
There were immediate technical problems:
- The oxygen permeability of lens materials of that era (hydrogel lenses) required either a very thin lens or very high water content. In reality, for safe extended wear, both were a necessity.
- Generally lenses made by competitive companies were made with the polymer in a dry state, then measured for accuracy, and finally hydrated to be ready for sterilisation and wear. The problem with this was the final hydration step. Hydration involves expansion. Thus any small error in dry state manufacture becomes exaggerated. Also the material hydration expansion factor may vary between batches. Since hydrated lenses are difficult to measure accurately, reproducibility can therefore be a significant problem.
- A thin, high water content lens tends to act like a sponge, dehydrating the cornea and making the lens uncomfortable with possible minor corneal damage.
The team was headed by Rod Watkins, with polymer technologist Tan Truong, physicist Rick Sweetman, optometrist Tony Phillips heading the clinical trials and, later, Carmel Copley in charge of microbiology testing. Clinical trials were carried out with animal trials at IMVS and human trials through Flinders Medical Centre (since FMC had American FDA recognition).
A huge amount of effort went into the designing of the molds. It was decided to use the spectacle lens mold concept i.e. a glass mold for the back surface and a polypropylene mold for the front. The other major and novel concept was the development of the ability to 'wet' mold lenses. This was a major step forward in that it eliminated the potential errors of the hydration step giving a more accurate lens at a reduced cost.
Developing a totally new concept - both in itself and also to a company new to the area - was, of course, not easy and was time-consuming. Nevertheless good progress was made with the molding process, the lens design, a name (XPR - Extended Wear Planned Replacement), and container design. Animal trials carried out by Tony Phillips at IMVS showed that the material was safe and 30 to 40 human patients wore the lens over a two year period.
A major concern in this novel start to disposable lenses was concern over patient compliance. Would patients actually dispose of their lenses at the end of a month or simply leave them in or attempt to re-use them after cleaning? Fortunately the problem of lens reuse occurred quite accidentally. The thin, high water content lens was fairly fragile. This meant that with anything more than the mildest attempt to clean the lens resulted in it splitting. With the later development of other disposable lens types, patient compliance has been somewhat of a problem (since they do not have built-in fragility) but not as bad as was feared in those early days
In spite of being several years ahead of the competition the project was abandoned around 1984. This arose for several reasons and must rank as one of the most disastrous decisions made by SOLA:
- Rod Watkins, the driving force behind the division, was forced to resign from the Board after a disagreement with CEO, John Heine. His replacement, Greg Rich, did not have Rod's optometric background and was heavily involved with take-overs in the USA.
- One of the American take-over companies was Barnes-Hind. Barnes-Hind was a major contact lens solution manufacturer and felt (unnecessarily) threatened by the concept of a disposable lens system that would obviate the need for their solutions. This had a major effect on the board having recently acquired Barnes Hind for Pilkington and who would be held responsible if the take-over failed.
- Incredibly at no stage was Tony Phillips, or even the lens wearers, consulted over the lens concept or its future potential. The decision to close the division down appears to have solely been taken at Board level.
By the close of the project it was very close to completion. Undoubtedly further improvement could have been made to the lens polymer and secondment of someone like Matthew Cuthbertson for a few months could have solved this. Perhaps the greatest compliment to the lens and its concept was that Johnson and Johnson in the USA brought out a similar lens around three to four years later and within a short time were producing half a billion dollars worth of lenses a year.
Nevertheless a small benefit did actually result from the years of effort and treasure expended. The quid pro quo for FMC providing the clinical and other facilities was that SOLA would fund the setting up and staffing, of a Medical Contact Lens Unit. This was to work with Professor Douglas Coster in the fitting of contact lenses in such cases as irregular or damaged corneas, hiding disfigured eyes, babies born with cataract (who could not have implant lenses), etc. In spite of the closure of the SOLA project the FMC Contact Lens Clinic survived as the first of its type in the country and is thriving some thirty years later, still run by Tony Phillips. In 1997 a similar clinic was established at The Adelaide Womens' and Childrens' Hospital, again the first of its type in the country.
THE SOLA CONTACT LENS PROJECT Rod Watkins
Up until the 1930s, contact lenses were made from blown glass. From then until the 1960s they were made from plastics, particularly polymethyl methacrylate. In 1964, contact lenses made from 2-hydroxy ethyl methacrylate (HEMA) became commercially available. This polymer could be hydrated to contain 38% water by weight, which had the advantages of carrying oxygen from the air to the eye and of flexibility which meant it was much more comfortable for the wearer. These lenses were spun cast in a concave mould. Over the next decade many companies began making these “soft” contact lenses. Most started with dehydrated buttons and lathe cut them on both surfaces, polished the edges, and then hydrated them. By 1975 a UK company, Duragel, was supplying HEMA buttons for lathe cutting to a great many contact lens laboratories. (Tony Phillips’ textbook Contact Lenses, Anthony J. Phillips and Lynne Speedwell, eds., 5th ed., 2007, Butterworth Heinemann, Edinburgh has excellent chapters on the history of contact lenses and on contact lens polymers.)
In 1976 I became interested in whether SOLA’s CR-39 casting technology could be adapted to cast finished contact lenses, with a view to substantially reducing the manufacturing cost. I applied for an Australian Government Industrial R&D Grant which was approved in March 1977, the first of three grants to support the project and the first of three general stages.
STAGE I
We set out to understand contact lens polymer chemistry and what a casting process would involve.
Polymer
Tan Truong, a PhD polymer chemist from the University of Adelaide, was employed. Tan had grown up in the Mekong delta in Vietnam and obtained a scholarship to study chemistry in Japan. He spoke no Japanese when he started there. He moved to Adelaide to complete his PhD, speaking no English when he arrived. His polymer work at SOLA was truly remarkable, but because it was not in the mainstream CR-39 business it was greatly under-recognised in the company.Tan began by obtaining HEMA monomer from every supplier around the world he could find. He then developed techniques for distilling the monomer, and used three different chromatography methods (at the University of Adelaide) for measuring the impurities.
He explored a large number of copolymers of HEMA and methacrylic acid to increase the water content beyond 38%, N-vinyl pyrollidone to increase the tensile strength, and various oxygen transmitters. I went to many international contact lens conferences to find out as much as I could about what other companies were doing, and scoured US patents for the state of the art.
Moulds
Rick Sweetman, a physicist, joined the project. About ten different mould materials of polymers, metal and glass and many mould designs were investigated. At the start these consisted of two injection moulded parts, but later an injection moulded concave mould and a metal electroformed nickel back mould were used, and later still a glass back mould. Cozzi Vozzo and Brian Adams in POD made the die inserts from hardened Stavax (a stainless steel) and the glass moulds.The very first moulds were acrylic but mould adhesion was a major problem, so we tried using Alkathene (a rubbery material), Teflon, various nylons and polypropylene. Many of these had good mould release but then were found to have unacceptable surface finish.

We were committed to closed-cell casting, partly to use the existing SOLA technology and partly to avoid some problems of spin casting, particularly the exposure of the back surface to the atmosphere during curing. This meant making gaskets less than 0.1mm thick. At the start, gaskets were punched from thin sheet but it was found to be more controllable to integrate the cavity spacing into the front mould.
Curing
This is where existing SOLA technology gave a head start. With the help of SOLA R&D, Process Control and production people, particularly Bob Sothman and later Huan Toh, methods of monomer preparation and many initiators, many cure cycles, and both air curing and water bath curing were investigated.

Lens
Lenses were first cast in dehydrated form and then hydrated, and later were cast already partly hydrated and then fully hydrated after mould release. Mould adhesion was a problem with casting dehydrated lenses, so the process developed into one of casting the lens at about 40% water content and then further hydrating it after opening. It was hard to completely bond the N-vinyl pyrollidone, and finished lenses were washed to remove residual NVP before being autoclaved for sterilisation.In mid-1978, as soon as the power was able to be controlled I began wearing SOLA contact lenses. This may have been the first clinical use of disposable contact lenses in the world.
STAGE II
Enough technology had been gathered by mid-1979 that we were encouraged to move the project forward. I obtained another Industrial R&D Grant with the goal of firming up the technology to a pre-production stage. Gerry Loots was brought back early from a European secondment to become General Manager of the Contact Lens Division. He took the project from the R&D phase to early production and also led the process development work.
Polymer
The HEMA monomer evaluation resulted in deciding to source from Mitsubishi Rayon in Japan, followed by distillation, washing and analysis by gas chromatography. The global expert on contact lens polymer chemistry, Brian Tighe, came from the UK and spent some time with Tan Truong advising on copolymer compositions and properties. At this time Tan was working on two alternatives, a lens hydrated to 38% in the mould and further hydrated to 65% after opening and one at 40% in the mould and 80% after opening.Moulds
When two moulds of the same material were used, the lens sometimes adhered to one mould and sometimes to the other. The final configuration used a concave polypropylene mould with a peripheral lip to act as a gasket. This mould had a reservoir to allow for overflow and also for shrinkage on polymerisation. It was flood filled and a glass back mould was dropped in and held in place with a weight that controlled the edge seal. The lens then always adhered to the convex glass mould, and a process was developed to release it from the mould in an alkaline solution before washing to remove the release agent. Henry Szymancyk developed some injection moulding changes that were key to raising the manufacturing yield from 15% to 85%.Curing
Cure cycles from 12 hours to 20 minutes were used. The resulting polymer was washed and the completeness of polymerisation and tensile strength were measured. The final cure cycle was fixed as a three hour air cure with a flat temperature profile. I don’t recall the temperature but I think it was fairly low, about 40°C. For a while Tan looked at polymerisation by ultraviolet radiation through the glass mould, with several different initiators.Lens
The final lens was polymerised at 40% water content and further hydrated to 80% after opening. (An Abbe refractometer was used to measure the water content and it was actually found to be about 3% higher at the front surface than the back, presumably because of the higher degree of polish on the back glass mould. This was an important advantage in oxygen transfer – see the patent and the comments by Gerry Loots below.) Minus lenses had a finished, fully hydrated, centre thickness of 0.08mm. The lenses were fairly fragile but the clinical work by Tony Phillips suggested that they could be safely handled by most wearers. This lens was chosen because the oxygen permeability was very high and we were beginning to focus on making disposable lenses for extended wear.There was a lot of work to get the edge finish smooth and consistent. We tried managing it through the mould design, and by casting lenses in dehydrated form and mechanically polishing the edges. Finally we worked with an external contractor to develop a method of laser polishing which showed promise. The clinical work by Tony Phillips showed that the edge finish was probably acceptable but this issue remained unresolved when the project ended.
Labels for the vials were printed (note the similarity to the SOLA Schultz-Crock ophthalmoscope labels) and it started to feel as if we had a product.
A patent was filed for this part of the project. Somewhat ironically, it was approved some time after the project ended.

Microbiology
Contact lenses were (and still are) classified as pharmaceuticals in the United States and are controlled by the Food and Drug Administration. I visited the FDA in Bethesda, Maryland, to talk about the requirements for clinical trials and microbiological testing, and the issues in carrying these out in Australia for approval in the US. In spite of their reputation, the FDA was extremely helpful and it was clear that there would be no problem in having them accept studies carried out by approved organisations in Australia. There were three parts to the FDA requirements- wearer trials, microbiology and toxicity. The microbiology was a routine procedure of incubating lenses and seeing what bugs grew, but the toxicity tests required fitting contact lenses to rabbits and measuring the reaction of the eyes. Initially we contracted Flinders Medical Centre to do the microbiological testing, but then Carmel Copley, a microbiologist, was employed by SOLA to do this work on site. The rabbit toxicity work was carried out by the Institute for Medical and Veterinary Science in Adelaide, so special lenses were made for that purpose and Tony Phillips fitted them.Metrology, SOPs
During this stage of the project, Standard Operating Procedures and Quality Standards were written for all stages of production and post-production testing, packaging and sterilisation. R&D records were formalised with detailed work logs, and regular detailed technical reports were filed. Major Technical Reports were produced on twelve different stages of the process. Equipment was bought for monomer analysis and for lens measurement, and equipment for lens tensile strength and oxygen permeability measurement was designed and built by SOLA.Production
A Class 100 clean room was built with high efficiency HEPA filters capable of filtering air particles down to 0.3 microns. It was a challenge to put a fume cabinet and casting ovens into the room without compromising the air quality. Entry was through a change area and all staff wore Tyvek clean room clothing and stepped on sticky mats before entry. The filling stations in the clean room were in laminar flow benches for additional particle filtration and the air purity was monitored regularly by outside consultants. The air temperature in the room was maintained at 20±1°C and the humidity at 35±5%. Gerry Loots recruited some of SOLA’s best production operators, particularly Debbie Reddington, and their standard was excellent.Clinical
The earliest clinical trials were contracted to the Cornea and Contact Lens Unit at the University of NSW. A protocol was developed for an extended wear trial, I think for about 50 wearers, and a contract signed. The person responsible for the trials, Michel Guillon, then visited SOLA and said that because the exchange rate between the AUD and the USD had changed the price had increased, but was unable to explain how the exchange rate affected the project costs. Although SOLA agreed to this demand, the planned trial was never completed. This may have been partly due to the fact that the CCLRU reported some wearers with red eyes and claimed a smell from the silicone rubber vial stoppers. We were unable to find any smell. A large number of stoppers were washed, the solution was analysed and found to be free of contaminants. As we were unable to find any contaminant, and the vials were sourced from a major pharmaceutical vial supplier, and no red eye reaction had been found in any of the SOLA in-house wearers (and no red eye reaction was found in the later trials by Tony Phillips or the rabbit toxicity tests) we believed the most likely source of the problem lay within the CCLRU. It is fair to say, however, that the CCLRU was half-hearted about the SOLA project before this and positively unsupportive afterwards. The head of the CCLRU, Brien Holden, told me later that at the time he saw no future for extended wear contact lenses.SOLA then had the good fortune to bring into Tony Phillips to the project. Tony was (and is) perhaps the world’s pre-eminent clinical expert in contact lenses. He had visited Australia several times to speak at professional meetings, and was attracted by the combination of R&D at SOLA, a formal appointment in the Department of Ophthalmology at Flinders University, and private practice. He fitted SOLA contact lenses to about 40 people and provided advice on optometrist and wearer matters and on acceptable quality standards.
At the end of this stage we were confident of being able to cast finished contact lenses on power, with high accuracy and with good control and repeatability of the process. The clinical work had convinced us that the future of the contact lens industry lay in extended wear. We had the very best people in R&D, in production, in testing and measurement and in clinical work. By the end of 1981 there were 14 people full-time on the project.
STAGE III
The Pilkington acquisition of SOLA dominated the project for the rest of its duration.
Greg Rich was appointed to head the project. He had little experience in managing R&D staff and as a result Tan Truong left SOLA. A Melbourne consultant, Kevin Luscombe, was contracted to develop marketing and promotional material. Focus groups of contact lens wearers were conducted in Melbourne and interviews were carried out with Tony Phillips and other optometrists in Adelaide by a contracted market research company, Zenith Research, to explore the appeal of extended wear lenses. (The Zenith Research reports were extremely positive.) Kevin Luscombe developed the brand name “XPR”. As disposable extended wear lenses were a new concept it was felt that advice was needed both for optometrists and for wearers, so practitioner and wearer protocols called the “SOLA System” were developed and the lens became the “SOLA System XPR”.
In this pre-production phase Gerry Loots headed the design of an innovative packaging system for retail use. Patents were applied for in Australia and Europe in November 1986 (Australian Patent Application 1986065647 and European Patent Application EP0269367A2).
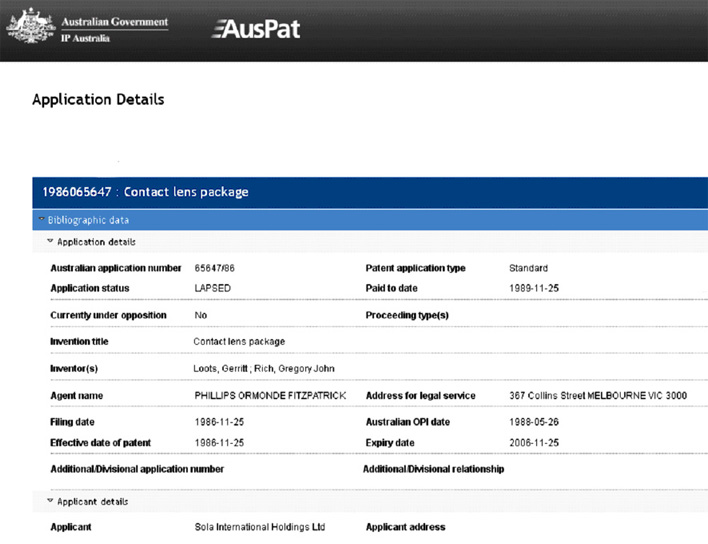
Pilkington wanted to be a significant player in the global contact lens industry. In 1980, Bob Jose went to the US and visited NPDC, the parent company of the Hydron group of contact lens companies and initiated discussions about acquisition. Hydron had 700 employees in seven countries and a projected turnover in 1983 of US$90 million.
I then visited American Hydron in New Jersey with Tom Jackson and Bob Rylance from Pilkington. A formal proposal between Pilkington and NPDC was reached in February 1981. NPDC agreed to sell 40% of American Hydron and Hydron Canada, and 60% of all off-shore companies, for an initial loan from Pilkington followed by US$35.5 million to be paid in three tranches. This method of entry would provide Pilkington with immediate access to the global market, to Hydron intellectual property and to FDA approval experience, and would provide a way to commercialise the SOLA technology.
I presented this proposal to the Pilkington Optical Division Board (ODB) in March 1982. The Board agreed to set up a sub-committee comprised of Leslie Wall (Chairman), Noel Roscrow, me, Bob Rylance and Ted Ellis to look at the proposal in detail and make a recommendation. Leslie Wall and Bob Rylance came to SOLA and the sub-committee met with the R&D staff, with Tony Phillips, with Laubman and Pank optometrists and with Flinders University (including the Professor of Ophthalmology, Doug Coster, and the microbiologists).
Leslie Wall reported back to the June 1982 meeting of the ODB the recommendations:
- that the NPDC proposal be accepted and the first tranche be paid
- that the acquisition of Hydron be focussed on exploiting the SOLA technology and on the global opportunity for extended wear contact lenses
- that Pilkington fund the SOLA project to relieve it of having to continue to find Australian Government grant funds
- that a high level international practitioner advisory group be funded.
These recommendations were accompanied by a detailed global business analysis and a detailed plan for a SOLA manufacturing plant to cast 1,500 lenses per shift that I had developed. This fully costed manufacturing facility was planned to be in production by late 1984 (with a direct cost of A$2.27 per lens) to support the Hydron acquisition.
The ODB agreed to make the loan to Hydron but deferred reaching a decision on either the acquisition or the SOLA proposal. Leslie Wall felt strongly about this, as he was the Pilkington Main Board representative on the ODB and chairman of the sub-committee making the recommendation, and as it turned out correctly believed this was a unique window of opportunity for Pilkington. This indecision by the ODB in June 1982 became critical in the future of SOLA and Pilkington Visioncare in contact lenses (see below). The proposal to Pilkington valued the whole Hydron group at US$67 million. The NPDC offer to Pilkington lapsed and the Hydron group was sold shortly after to another company for US$120 million.
The SOLA project was then left without a strategy for commercialisation and as a result, in September 1982, I produced a detailed proposal that SOLA fund from its own resources a small pilot manufacturing plant costing $400,000 over two years and capable of initially producing 200 lenses per day. It included a plan for self-generated funding to grow to 2,000 lenses per day with 60 staff.
John Heine was enthusiastic about this but wanted to proceed on a larger scale, so he proposed to the ODB a $2.25 million plan to make and test market disposable extended wear lenses in Australia and Singapore. This proposal was also not supported.
THE END OF THE PROJECT
When I left SOLA in 1983, Pilkington was pursuing other options for entry into the contact lens market. This resulted firstly in the acquisition of Syntex Ophthalmics Inc., a Phoenix, Arizona contact lens company, for US$60 million in November 1985. The company was renamed Sola-Syntex Ophthalmics, Inc. Pilkington then acquired Paragon Optical, a small contact lens company based in Mesa, Arizona, as a result of a patent infringement lawsuit. In 1987, Pilkington acquired from Revlon two companies, Barnes Hind (a contact lens and contact lens solution company) and Coburn Optical, for a total of £386 million (USD 580 million). Barnes Hind was initially named Sola-Barnes Hind, and later was renamed Pilkington Barnes Hind. The CEO of this company was initially Edwin Harless and later Gary Mulloy.
Gerry Loots adds the following on Syntex and Barnes Hind:
When SOLA acquired Syntex, the CEO who was appointed was not allowed to take up his role for 6 months or so due to contractual issues as he worked for Bausch & Lomb who evoked a non-compete clause. John Heine actually stayed in Phoenix, Arizona for a while to run Syntex before Dick Kapash from SOLA USA was put in charge and subsequently replaced by Terry Smith.
The R&D team at Syntex were much more experienced and much better resourced than the Adelaide team. It turned out Syntex were developing a similar product to XPR. I visited Syntex for a couple of weeks on a technical exchange. I was amazed at how close the two processes were. My conclusion was that we had the better, more unique lens because we had proven that the material and mould material combinations actually created an environment in which the tears seemed to pump through the lens to keep the back surface hydrated. This was studied a great deal by Dr Nathan Efron. To this day I don’t understand why that part of the work was not developed further.
When Pilkington acquired Barnes Hind in 1987 the market dynamics changed. We suddenly had an extra 15% of the contact lens market in the US plus Syntex’s about 8 %; we were a big player. The market was in the USA so there is logic in doing your R&D there and the assessment was that the Barnes Hind R & D group knew what they were doing with contact lenses. So XPR was wound up.
I have been told that the CEO of Syntex visited Lonsdale to meet with John Heine, that the visit lasted only half a day and that he did not visit the contact lens area. After the meeting John Heine told the staff that the project was closed.
The people in R&D, production and clinical areas who had worked so hard for success over such a long period held a wake for the project. Some contact lenses were incinerated to represent the ashes of the SOLA XPR contact lens project. Here is one.
The SOLA XPR project was wound up in 1986. At the time Johnson and Johnson, Bausch and Lomb and Barnes Hind were all working on disposable extended wear lenses, although all started several years behind SOLA. Johnson and Johnson bought Danalens, a small Danish company with extended wear lens technology, in 1984 and began marketing Vistakon Acuvue disposable lenses for one-week extended wear in six-packs in 1987.
Bausch and Lomb launched SeeQuence replaceable lenses in 1989.
When Pilkington acquired Barnes Hind in 1987, the company was working on an extended wear lens and so an effort was made to retrieve the XPR records. It was discovered that all of the R&D and production files had been discarded, as the filing cabinets were needed for another purpose. In 1989 Pilkington–Barnes Hind launched the 78% water content “Calendar” lens, a lens very similar to the XPR, for monthly replacement.
Barnes Hind however failed to live up to its financial projections and in November 1995, Pilkington sold the Barnes Hind contact lens solutions business to Allergan for £50 million (and in 1995 also sold Paragon Optical). In October 1996 the Barnes Hind contact lens business, the last remaining vestige of the Pilkington Visioncare Division, was sold to Wesley Jessen for $62.4 million.
In hindsight it seems likely that the opportunity for SOLA was lost not in 1986 but as early as 1982 when the Pilkington Optical Division Board failed to support the recommendations to acquire Hydron and commercialise the SOLA technology of the time. By 1989 the ability to shape the global market was largely lost. It remains unclear why Pilkington did not pursue either the Hydron opportunity or the SOLA proposals of 1982.
 Home
Home Table of Contents
Table of Contents